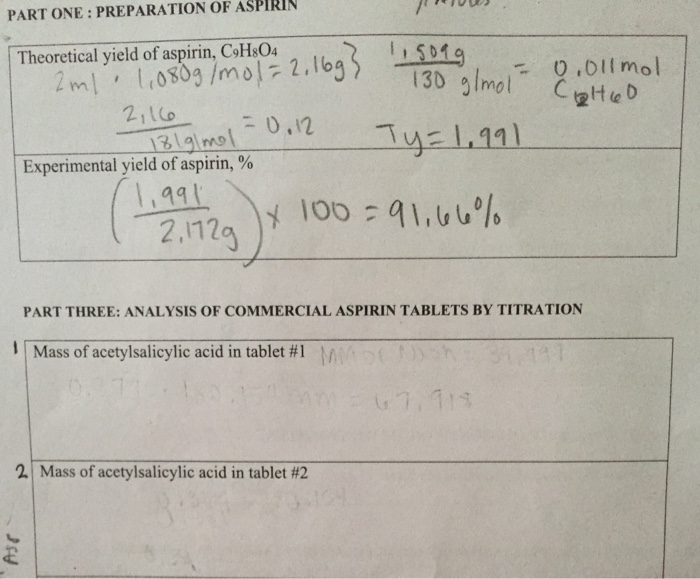
Calculate the theoretical yield of aspirin
Calculate The Theoretical Yield Of Aspirin. If a team obtains 1.85 g of asprin product form, what was the percent yield? The results displayed a percent yield of 34.3%, from a theoretical yield of about 1.97g of aspirin and an actual yield of approximately 0.68g of aspirin.upon completion of the lab, analysis, and calculations, it is evident that the synthesis of aspirin is possible using these methods but that the yield will be relatively low. Thus, the calculated value of crude synthesized aspirin was 3.029 grams. The actual yield would be the observed mass of.

Theoretical yield = moles of the product obtained/moles of the. Mass of filter paper (g): Calculate the percent yield for the above reaction if the amount of aspirinobtained was 2.301 g. Limiting reaction is salicylic acid since it has less moles. % yield of aspirin % yield= actual yield/theoretical yield * 100. The fraction of the theoretical yield is calculated by using the actual experimentally obtained amount of product and dividing it by the amount expected in theory.
Thank you calculate the theoretical yield of the aspirin.
Actual yield of aspirin in lab after filtered/dried (mock value) = 370 g mass of theoretical yield of aspirin (mock value) = 360.3 g percent yield = (actual ÷ theoretical) x. Thus, the calculated value of crude synthesized aspirin was 3.029 grams. We calculate the theoretical yield of aspirin for the quantity mole s of salicylic acid. Calculate the percent yield if the amount of aspirin obtained was 2.301 g ? Following purification, the calculated mass of the final aspirin product was 2.169 grams. Calculated theoretical yield of &.so aspirin product:

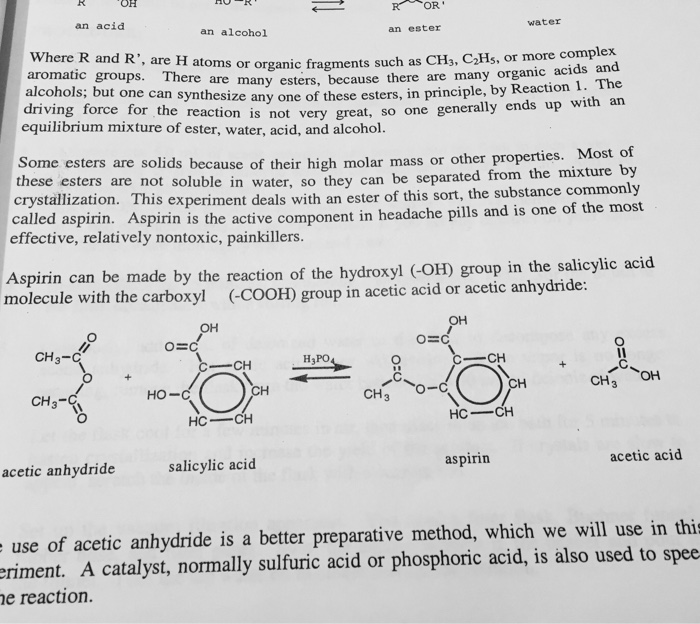
Calculate the percent yield for the above reaction if the amount of aspirinobtained was 2.301 g. If a team obtains 1.85 g of asprin product form, what was the percent yield? Determine the theoretical yield of aspirin for the reaction constants i pe riodic table cetic aspir anhydride (cho3with salicylic acid (c h os) to form aspirin (cghgo4) and acetic acid (hc2 h3 o2) the balanced equation is is shown here in can be made in the laboratory by reacting a in a laboratory synthesis, a student begins with 2.90 ml of acetic. And here�s how you calculate percent yield. Excess of acetic anhydride ?
 Source: seikyusho.jp
Source: seikyusho.jp
= (weight of the product obtained/molar mass of the. Therefore the theoretical yield of acetylsalicylic acid is 0014 moles. If a team obtains 1.85 g of asprin product form, what was the percent yield? So there are 0.01448 moles of aspirin? % yield of aspirin % yield= actual yield/theoretical yield * 100.
 Source: cityraven.com
Source: cityraven.com
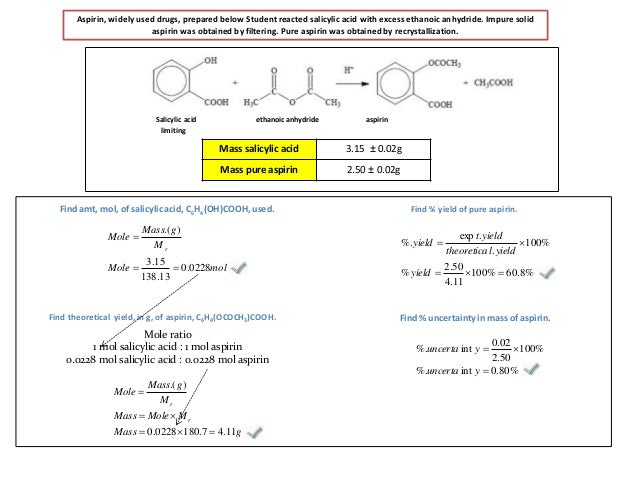
M r aspirin = (9 x 12) + (8 x 1) + (4 x 16) = 180. Thus, the calculated value of crude synthesized aspirin was 3.029 grams. Calculate the percent yield for the above reaction if the amount of aspirinobtained was 2.301 g. Theoretical yield 2.0 g salicylic acid (1 mole/138.0 g) = 0.014 moles Calculate the percent yield if the amount of aspirin obtained was 2.301 g ?

Actual yield of aspirin in lab after filtered/dried (mock value) = 370 g mass of theoretical yield of aspirin (mock value) = 360.3 g percent yield = (actual ÷ theoretical) x. If the synthesis reaction was started with 1.00 g of salicylic acid, what volume of acetic anhydride would Is taken in reaction)x 100 %. Theoretical yield = moles of the product obtained/moles of the. The fraction of the theoretical yield is calculated by using the actual experimentally obtained amount of product and dividing it by the amount expected in theory.
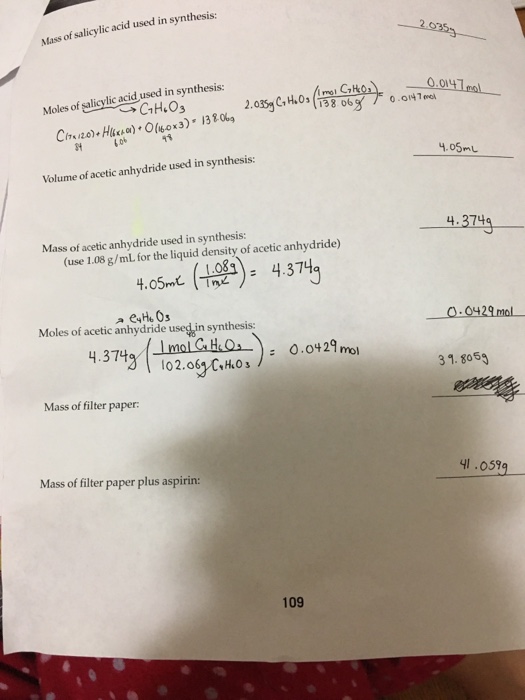
Yield actual yield 100 theoretical yield 3. Mole ratio is one to one. Therefore the theoretical yield of acetylsalicylic acid is 0014 moles. Theoretical yield 2.0 g salicylic acid (1 mole/138.0 g) = 0.014 moles The fraction of the theoretical yield is calculated by using the actual experimentally obtained amount of product and dividing it by the amount expected in theory.
M r aspirin = (9 x 12) + (8 x 1) + (4 x 16) = 180. Reactant taken (the limiting reactant i. If the theoretical yield of aspirin is 2.748 g and you obtained 2.47 g of aspirin, what is your percent yield? Yield actual yield 100 theoretical yield 3. Calculated theoretical yield of &.so aspirin product:

The mass of aspirin and filter paper was 3.159 grams. Excess of acetic anhydride ? If the theoretical yield of aspirin is 2.748 g and you obtained 2.47 g of aspirin, what is your percent yield? Following purification, the calculated mass of the final aspirin product was 2.169 grams. The purpose of this experiment was to learn how to synthesize aspirin and accurately determine the theoretical and percent yield of the aspirin.
And here�s how you calculate percent yield. The density of acetic anhydride is 1.08 g/ml (hint: The results displayed a percent yield of 34.3%, from a theoretical yield of about 1.97g of aspirin and an actual yield of approximately 0.68g of aspirin.upon completion of the lab, analysis, and calculations, it is evident that the synthesis of aspirin is possible using these methods but that the yield will be relatively low. Mass of aspirin 261 grams theoretical yield. The actual yield would be the observed mass of.
 Source: chegg.com
Source: chegg.com
The density of acetic anhydride is 1.08 g/ml (hint: 2figure 2 synthesis of aspirin in this experiment you will measure the amount of. Calculate the percent yield for the above reaction if the amount of aspirinobtained was 2.301 g. 3.26 salycylic acid color with fer fecl, color tests your aspirin color with fecl: Once the aspirin was prepared, it was isolated from the reaction.

If a team obtains 1.85 g of asprin product form, what was the percent yield? Write out the balanced equation for the reaction between salicylic acid and aceticanhydride.2. 3.26 salycylic acid color with fer fecl, color tests your aspirin color with fecl: The mass of the filter paper was.1300 grams. Excess of acetic anhydride ?
 Source: chegg.com
Source: chegg.com
Excess of acetic anhydride ? So for one mole of salicylic acid used one mole of aspirin is used too. The fraction of the theoretical yield is calculated by using the actual experimentally obtained amount of product and dividing it by the amount expected in theory. Mole ratio is one to one. The mass of the filter paper was.1300 grams.
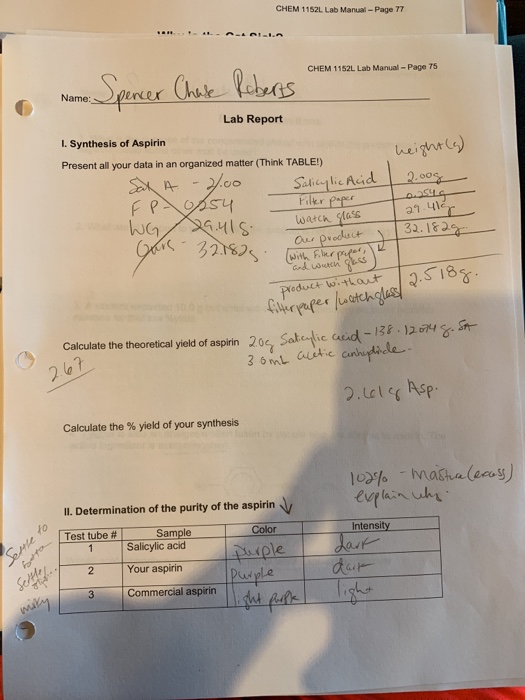
So for one mole of salicylic acid used one mole of aspirin is used too. From the equation, moles of salicylic acid = moles of aspirin. Here�s how to calculate the theoretical yield of aspirin. We calculate the theoretical yield of aspirin for the quantity mole s of salicylic acid. 3.26 salycylic acid color with fer fecl, color tests your aspirin color with fecl:
 Source: homeworklib.com
Source: homeworklib.com
Mole ratio is one to one. If a team obtains 1.85 g of asprin product form, what was the percent yield? Product)/ (weight of the reactant taken/molar mass of the reactant)x. The synthesis occurs due to a reaction between salicylic acid and acetic anhydride. % yield of aspirin % yield= actual yield/theoretical yield * 100.
 Source: chegg.com
Source: chegg.com
Solution #percent yield = actual yield/theoretical yield × 100 % = (2.47 color(red)(cancel(color(black)(g))))/(2. If a team obtains 1.85 g of asprin product form, what was the percent yield? Calculate the percent yield if the amount of aspirin obtained was 2.301 g ? Theoretical yield can be given as. Calculate the theoretical yield of aspirin if you start with 2.687 g of salicylic acidand an excess of acetic anhydride.
 Source: chegg.com
Source: chegg.com
Here�s how to calculate the theoretical yield of aspirin. So for one mole of salicylic acid used one mole of aspirin is used too. The purpose of this experiment was to learn how to synthesize aspirin and accurately determine the theoretical and percent yield of the aspirin. 2.50x 180 aspirin data table for aspirin: Determine the theoretical yield of aspirin for the reaction constants i pe riodic table cetic aspir anhydride (cho3with salicylic acid (c h os) to form aspirin (cghgo4) and acetic acid (hc2 h3 o2) the balanced equation is is shown here in can be made in the laboratory by reacting a in a laboratory synthesis, a student begins with 2.90 ml of acetic.
 Source: rgops.com
Source: rgops.com
If you started with exactly 1.885 g of salicylic acid and 3.50 ml of acetic anhydride, calculate the theoretical yield of asprin. We calculate the theoretical yield of aspirin for the quantity mole s of salicylic acid. Is taken in reaction)x 100 %. Here�s how to calculate the theoretical yield of aspirin. Mass of filter paper (g):

And here�s how you calculate percent yield. Theoretical yield of aspirin therefore = 0.1 moles. Thus, the calculated value of crude synthesized aspirin was 3.029 grams. **the molar masses of the salicylic acid and the acetylsalicylic acid will need to be calculated using the periodic table before finding the theoretical yield. = (weight of the product obtained/molar mass of the.

If the synthesis reaction was started with 1.00 g of salicylic acid, what volume of acetic anhydride would If a team obtains 1.85 g of asprin product form, what was the percent yield? The actual yield would be the observed mass of. 32 g but the lab cheese moisture having identified silver nitrate as the limiting reagent we can do the rest of the calculation: Mole ratio is one to one.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title calculate the theoretical yield of aspirin by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.