How many protons are in iron
How Many Protons Are In Iron. How many protons does fe 3? It is a metal that belongs to the first transition series and group 8 of the periodic table.it is, by mass, the most common element on earth, right in front of oxygen (32.1% and 30.1%, respectively), forming much of earth�s outer and inner core.it is the fourth most common. How many protons and electrons are in an fe3+ ion? In the nucleus are protons and neutrons.
 Manganese by Dominic Mielke From haikudeck.com
Manganese by Dominic Mielke From haikudeck.com
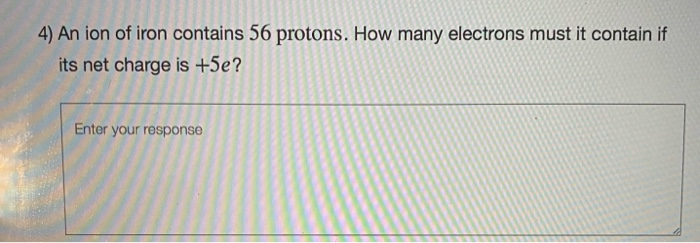
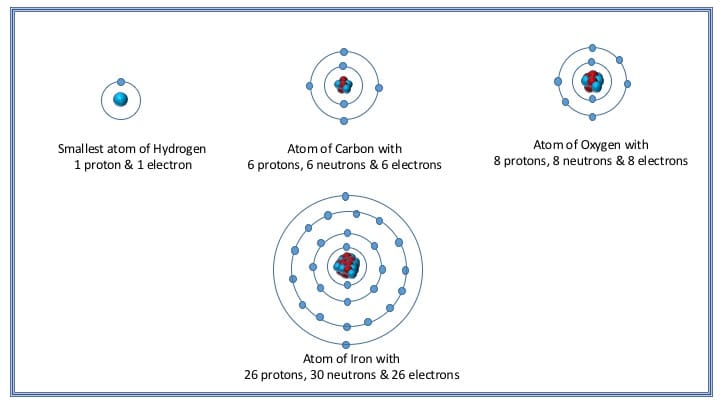
An iron 56 nucleus contains a 56 protons and 56 neutrons b 26 protons and 30 neutrons c 56 protons and 26 neutrons d 30 protons and 26 neutrons e 26 protons and 26 neutrons The 3+ charge on the ion means that the ion has lost 3 electrons, therefore the number of protons is 26 (23+3). A neutral iron atom contains 26 protons and 30 neutrons plus 26 electrons in four different shells around the nucleus. An iron atom contains 26 electrons. How many protons and electrons are in an fe3+ ion? It is a metal that belongs to the first transition series and group 8 of the periodic table.it is, by mass, the most common element on earth, right in front of oxygen (32.1% and 30.1%, respectively), forming much of earth�s outer and inner core.it is the fourth most common.
It is a metal that belongs to the first transition series and group 8 of the periodic table.it is, by mass, the most common element on earth, right in front of oxygen (32.1% and 30.1%, respectively), forming much of earth�s outer and inner core.it is the fourth most common.
It is by mass the most common element on earth, forming much of earth’s outer and inner core. Iron (/ ˈ aɪ ər n /) is a chemical element with symbol fe (from latin: Zinc has 30 protons, 35 neutrons and 30 electrons: These consist of a varying number of electrons but the same number of protons and neutrons. And after this, this can be a initial photograph. How many protons and electrons does iron ion have?
 Source: reviewhomedecor.co
Source: reviewhomedecor.co
Again, the iron atom donates two electrons in the 4s orbital and an electron in the 3d orbital to convert an iron ion (fe 3+ ). For this, iron ion (fe 2+) has a total of fourteen valence electrons. Ferrum) and atomic number 26. 26 massive, positively charged nuclear particles, this demands that there are 30 neutrons in this isotope. The 3+ charge on the ion means that the ion has lost 3 electrons, therefore the number of protons is 26 (23+3).
 Source: material-properties.org
Source: material-properties.org
26 is the atomic number for iron. For zinc, the atomic weight is 65.39, so the mass number is closest to 65. An iron 56 nucleus contains a 56 protons and 56 neutrons b 26 protons and 30 neutrons c 56 protons and 26 neutrons d 30 protons and 26 neutrons e 26 protons and 26 neutrons Since atomic number of iron is equal to 26, therefore, number of protons in iron is equal to 26. How many protons are there in an atom of iron?
 Source: awesomehome.co
Source: awesomehome.co
This means that iron has 26 protons. Iron is a metal in the first transition series. Fe³⁺, on the other hand, is the ion resulting from the loss. An iron 56 nucleus contains a 56 protons and 56 neutrons b 26 protons and 30 neutrons c 56 protons and 26 neutrons d 30 protons and 26 neutrons e 26 protons and 26 neutrons 26 is the atomic number for iron.
 Source: dantuckerautos.com
Source: dantuckerautos.com
A circular shell is located outside of the nucleus and contains electrons that are equal to protons. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z….stable isotopes. 26 is the atomic number for iron. Iron exists in its ionic form as either fe²⁺ or fe³⁺. A circular shell is located outside of the nucleus and contains electrons that are equal to protons.
 Source: whatisnuclear.com
Source: whatisnuclear.com
Ferrum) and atomic number 26. Copper has 29 protons, 35 neutrons and 29 electrons: 13 rows iron is the 26th element of the periodic table so its atomic number is 26. Hare, the electron configuration of iron ion (fe 3+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 5. Iron (/ ˈ aɪ ər n /) is a chemical element with symbol fe (from latin:
 Source: chegg.com
Source: chegg.com
Gallium has 31 protons, 39 neutrons and 31 electrons: 26 massive, positively charged nuclear particles, this demands that there are 30 neutrons in this isotope. How many protons are there in iron 57? Iron is a chemical element with atomic number 26 which means there are 26 protons in its nucleus. Hare, the electron configuration of iron ion (fe 3+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 5.
 Source: reviewhomedecor.co
Source: reviewhomedecor.co
The chemical symbol for iron is fe. The number of protons in a given atom is known as the atomic number. For hydrogen, 1.008 is closer to 1 than 2, so let�s call it 1. Iron has 26 protons, 30 neutrons and 26 electrons: 26 massive, positively charged nuclear particles, this demands that there are 30 neutrons in this isotope.
 Source: chegg.com
Source: chegg.com
And after this, this can be a initial photograph. The atomic number #z# of #fe# #=26#. Again, the iron atom donates two electrons in the 4s orbital and an electron in the 3d orbital to convert an iron ion (fe 3+ ). Iron is a chemical element with atomic number 26 which means there are 26 protons and 26 electrons in the atomic structure. Given that the nucleus contains 26 protons, i.e.
 Source: clutchprep.com
Source: clutchprep.com
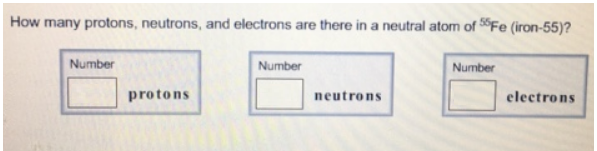
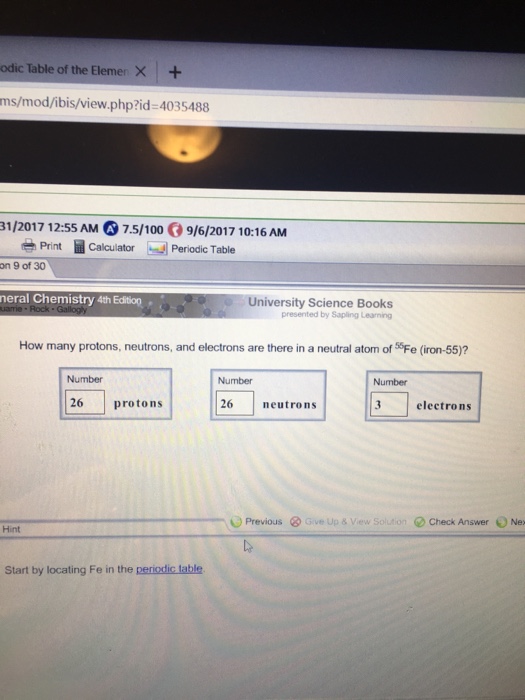
13 rows iron is the 26th element of the periodic table so its atomic number is 26. Ferrum) and atomic number 26. 26 is the atomic number for iron. How many protons are there in an atom of iron? A neutral iron atom contains 26 protons and 30 neutrons plus 26 electrons in four different shells around the nucleus.
 Source: putney-lettings.blogspot.com
Source: putney-lettings.blogspot.com
26 massive, positively charged nuclear particles, this demands that there are 30 neutrons in this isotope. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z….stable isotopes. Zinc has 30 protons, 35 neutrons and 30 electrons: For zinc, the atomic weight is 65.39, so the mass number is closest to 65. Since atomic number of iron is equal to 26, therefore, number of protons in iron is equal to 26.
 Source: dantuckerautos.com
Source: dantuckerautos.com
For this, iron ion (fe 2+) has a total of fourteen valence electrons. It is by mass the most common element on earth, forming much of earth’s outer and inner core. Given that the nucleus contains 26 protons, i.e. Iron exists in its ionic form as either fe²⁺ or fe³⁺. Iron is a chemical element with atomic number 26 which means there are 26 protons in its nucleus.
 Source: dantuckerautos.com
Source: dantuckerautos.com
How many protons are there in iron 57? Ferrum) and atomic number 26. The atomic number #z# of #fe# #=26#. How many protons are in an iron 61 atom? 13 rows iron is the 26th element of the periodic table so its atomic number is 26.
In the nucleus are protons and neutrons. Hare, the electron configuration of iron ion (fe 3+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 5. Fe³⁺, on the other hand, is the ion resulting from the loss. 13 rows iron is the 26th element of the periodic table so its atomic number is 26. Ferrum) and atomic number 26.
 Source: selftution.com
Source: selftution.com
Because for every exam in chemistry and physics you ever sit, you should quite properly be supplied with a periodic table. For this, iron ion (fe 2+) has a total of fourteen valence electrons. These consist of a varying number of electrons but the same number of protons and neutrons. Gallium has 31 protons, 39 neutrons and 31 electrons: Nickel has 28 protons, 31 neutrons and 28 electrons:
 Source: chegg.com
Source: chegg.com
Iron (/ ˈ aɪ ər n /) is a chemical element with symbol fe (from latin: Iron has 26 protons, 30 neutrons and 26 electrons: Atomic number of an element=number of electrons=number of protons. 1 answer anor277 jul 21, 2016 why #26#! Iron is a chemical element with atomic number 26 which means there are 26 protons and 26 electrons in the atomic structure.
 Source: haikudeck.com
Source: haikudeck.com
Cobalt has 27 protons, 32 neutrons and 27 electrons: For zinc, the atomic weight is 65.39, so the mass number is closest to 65. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z….stable isotopes. Because for every exam in chemistry and physics you ever sit, you should quite properly be supplied with a periodic table. Iron exists in its ionic form as either fe²⁺ or fe³⁺.
 Source: pinterest.com
Source: pinterest.com
How many protons are there in iron 57? Gallium has 31 protons, 39 neutrons and 31 electrons: The number of protons in a given atom is known as the atomic number. For this, iron ion (fe 2+) has a total of fourteen valence electrons. Nickel has 28 protons, 31 neutrons and 28 electrons:
 Source: dantuckerautos.com
Source: dantuckerautos.com
It is by mass the most common element on earth, forming much of earth’s outer and inner core. Ferrum) and atomic number 26. These consist of a varying number of electrons but the same number of protons and neutrons. How many protons in iron. Hare, the electron configuration of iron ion (fe 3+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 5.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many protons are in iron by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






