How many protons are in sulfur
How Many Protons Are In Sulfur. And thus finally, if we have the 32s2− ion, there are 18 electrons, 16 protons, and 16 neutrons. There are 16protons and 16 neutrons present in sulphur element. The easy way is to write the electron configuration. Sulfur is the 16th element in the periodic table.
 Bohr Diagram Of Sulfur From wiringall.com
Bohr Diagram Of Sulfur From wiringall.com
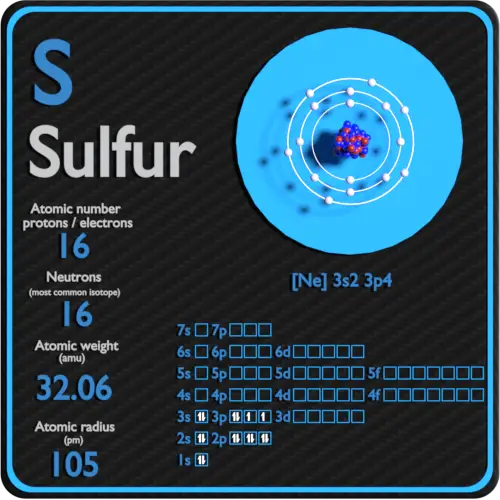
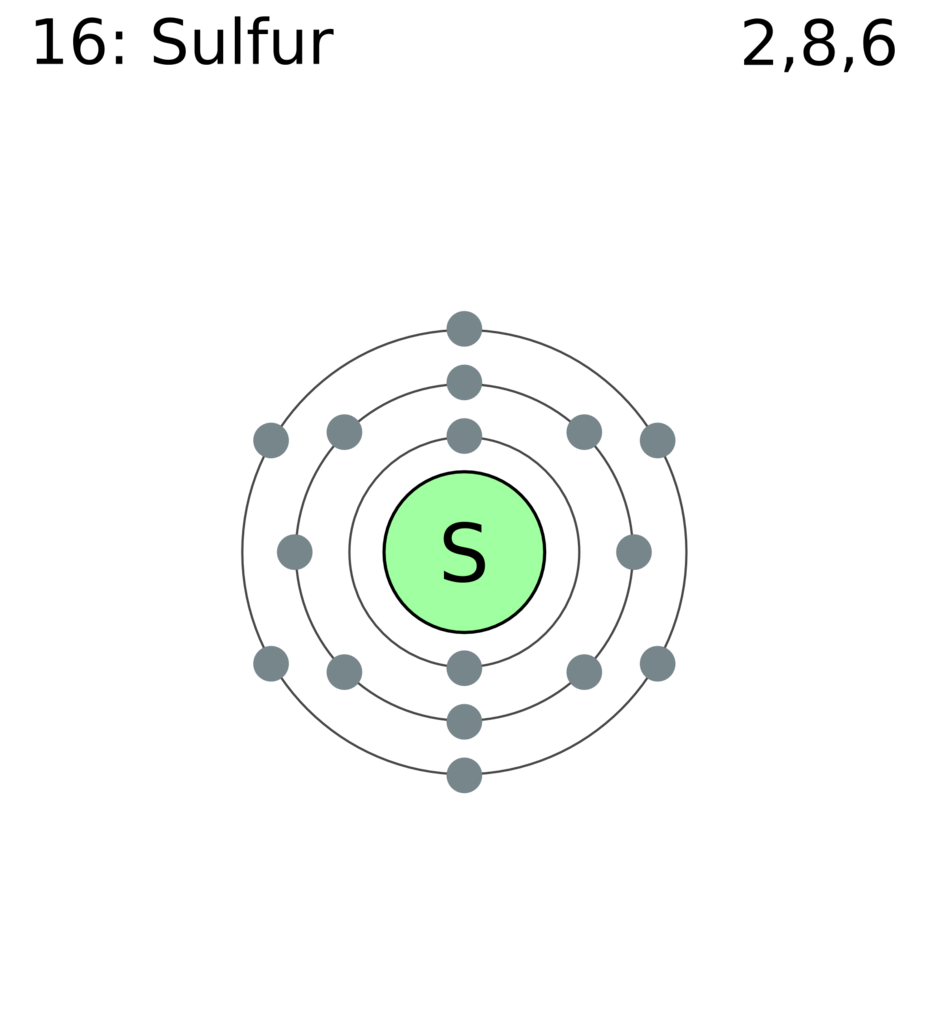
Sulphur has atomic number 16 which means that it has 16 protons. We can determine the total number of protons and neutrons present in any element by seeing it�s atomic number of that element. So it depends on the isotope. Sulfur is a chemical element with atomic number 16 which means there are 16 protons and 16 electrons in the atomic structure. There is an easy path to find valence electron which are located in the last shell of sulfur. Sulfur is abundant, multivalent, and nonmetallic.
How many protons and electrons are in atoms of sulfur atom?
How many protons nutrons and electrons does sulfur have? How many protons does sulfur have? The chemical symbol for sulfur is s. 2 protons 16 neutrons b. Thus, each atom or ion of sulfur must contain 16 protons. Sulfur is abundant, multivalent, and nonmetallic.
 Source: wiringall.com
Source: wiringall.com
Sulfur is a chemical element with atomic number 16 which means there are 16 protons and 16 electrons in the atomic structure. We have 16 nuclear protons, so there must be 17 neutrons , i.e. The easy way is to write the electron configuration. However, different isotopes of sulfur has number of neutrons ranging from 10 to 33. We are told that the ion also has 16 neutrons, meaning the mass number of the ion is 16 + 16 = 32.

We can determine the total number of protons and neutrons present in any element by seeing it�s atomic number of that element. So it depends on the isotope. Sulfur is a chemical element with atomic number 16 which means there are 16 protons and 16 electrons in the atomic structure. Protons are the permanent core particles of an. The number of protons and neutrons is 16 protons and 16 neutrons.

The atomic number, z, specifies the number of protons, positively charged, massive, fundamental particles present in the sulfur nucleus. However, different isotopes of sulfur has number of neutrons ranging from 10 to 33. 13 rows how many protons does a sulfur atom have? How many protons neutrons and electrons are in sulfur 32? How many protons and neutrons does sulfur have?
![]() Source: sciencenotes.org
Source: sciencenotes.org
There is an easy path to find valence electron which are located in the last shell of sulfur. For example in case of sulphur element there are 16 protons and neutrons present situation ce its atomic number is 16 (s16). 16 sulfur/atomic number because electrons have negligible mass, to account for the mass of the isotope, there must be 16 neutrons, 16 neutrally charged, massive, fundamental particles present in the sulfur nucleus. How many protons neutrons and electrons are in sulfur 32? Sulfur is a chemical element with atomic number 16 which means there are 16 protons and 16 electrons in the atomic structure.
 Source: ansaroo.com
Source: ansaroo.com
The atomic number, z, specifies the number of protons, positively charged, massive, fundamental particles present in the sulfur nucleus. And thus finally, if we have the 32s2− ion, there are 18 electrons, 16 protons, and 16 neutrons. How many protons and neutrons does sulfur have? Sulfur is the 16th element in the periodic table. We are told that the ion also has 16 neutrons, meaning the mass number of the ion is 16 + 16 = 32.
 Source: bigstockphoto.com
Source: bigstockphoto.com
What is the number of electrons in sulfur? Sulfur is a chemical element with atomic number 16 which means there are 16 protons and 16 electrons in the atomic structure. The easy way is to write the electron configuration. 13 rows how many protons does a sulfur atom have? We have 16 nuclear protons, so there must be 17 neutrons , i.e.
 Source: wiringall.com
Source: wiringall.com
Sulfur is a chemical element with atomic number 16 which means there are 16 protons and 16 electrons in the atomic structure. Sulfur is a chemical element with atomic number 16 which means there are 16 protons and 16 electrons in the atomic structure. 13 rows how many protons does a sulfur atom have? 2 protons 16 neutrons b. Sulfur is abundant, multivalent, and nonmetallic.
 Source: shutterstock.com
Source: shutterstock.com
2 protons 16 neutrons b. How many protons and electrons are in atoms of sulfur atom? What is the number of electrons in sulfur? Does sulfur have 17 neutrons? How many protons and neutrons does sulfur have?
 Source: elchoroukhost.net
Source: elchoroukhost.net
And thus finally, if we have the 32s2− ion, there are 18 electrons, 16 protons, and 16 neutrons. So it depends on the isotope. 18 protons and electrons and 22 neutrons. How many protons nutrons and electrons does sulfur have? 13 rows how many protons does a sulfur atom have?
 Source: dreamstime.com
Source: dreamstime.com
This number defines the sulfur atom as a sulfur atom (i.e if there were 17 protons, the atom would be chlorine). There is an easy path to find valence electron which are located in the last shell of sulfur. 2 protons 16 neutrons b. How many protons neutrons and electrons are in sulfur 32? All sulfur atoms have 16 protons because the number of protons determines what element it is.
 Source: material-properties.org
Source: material-properties.org
How many protons and neutrons does sulfur have? We have 16 nuclear protons, so there must be 17 neutrons , i.e. And thus finally, if we have the 32s2− ion, there are 18 electrons, 16 protons, and 16 neutrons. Thus, each atom or ion of sulfur must contain 16 protons. 18 protons and electrons and 22 neutrons.
 Source: ansaroo.com
Source: ansaroo.com
The number of protons and neutrons is 16 protons and 16 neutrons. What is the number of electrons in sulfur? We have 16 nuclear protons, so there must be 17 neutrons , i.e. There is an easy path to find valence electron which are located in the last shell of sulfur. The chemical symbol for sulfur is s.
 Source: genius.com
Source: genius.com
For example in case of sulphur element there are 16 protons and neutrons present situation ce its atomic number is 16 (s16). The number of protons and neutrons is 16 protons and 16 neutrons. Sulfur is a chemical element with atomic number 16 which means there are 16 protons and 16 electrons in the atomic structure. It means that sulfur atom has 16 protons and 16 electrons. 2 protons 16 neutrons b.
 Source: pinterest.com
Source: pinterest.com
Thus, each atom or ion of sulfur must contain 16 protons. We have 16 nuclear protons, so there must be 17 neutrons , i.e. How many protons nutrons and electrons does sulfur have? And thus finally, if we have the 32s2− ion, there are 18 electrons, 16 protons, and 16 neutrons. Protons are the permanent core particles of an.
 Source: slideshare.net
Source: slideshare.net
There is an easy path to find valence electron which are located in the last shell of sulfur. Protons are the permanent core particles of an. However, different isotopes of sulfur has number of neutrons ranging from 10 to 33. How many protons and electrons are in atoms of sulfur atom? How many protons does sulfur have?
 Source: schematron.org
Source: schematron.org
We are told that the ion also has 16 neutrons, meaning the mass number of the ion is 16 + 16 = 32. There are 16protons and 16 neutrons present in sulphur element. It means that sulfur atom has 16 protons and 16 electrons. Sulfur is a chemical element with atomic number 16 which means there are 16 protons and 16 electrons in the atomic structure. How many protons nutrons and electrons does sulfur have?
 Source: sciencephoto.com
Source: sciencephoto.com
How many protons and neutrons does sulfur have? How many protons nutrons and electrons does sulfur have? Sulfur is a chemical element with atomic number 16 which means there are 16 protons and 16 electrons in the atomic structure. The number of protons and neutrons is 16 protons and 16 neutrons. This number defines the sulfur atom as a sulfur atom (i.e if there were 17 protons, the atom would be chlorine).
 Source: colourbox.com
Source: colourbox.com
It means that sulfur atom has 16 protons and 16 electrons. How many protons neutrons and electrons in 40 ar? 2 protons 16 neutrons b. All sulfur atoms have 16 protons because the number of protons determines what element it is. The atomic number, z, specifies the number of protons, positively charged, massive, fundamental particles present in the sulfur nucleus.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many protons are in sulfur by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.