Lewis dot structure for hco3
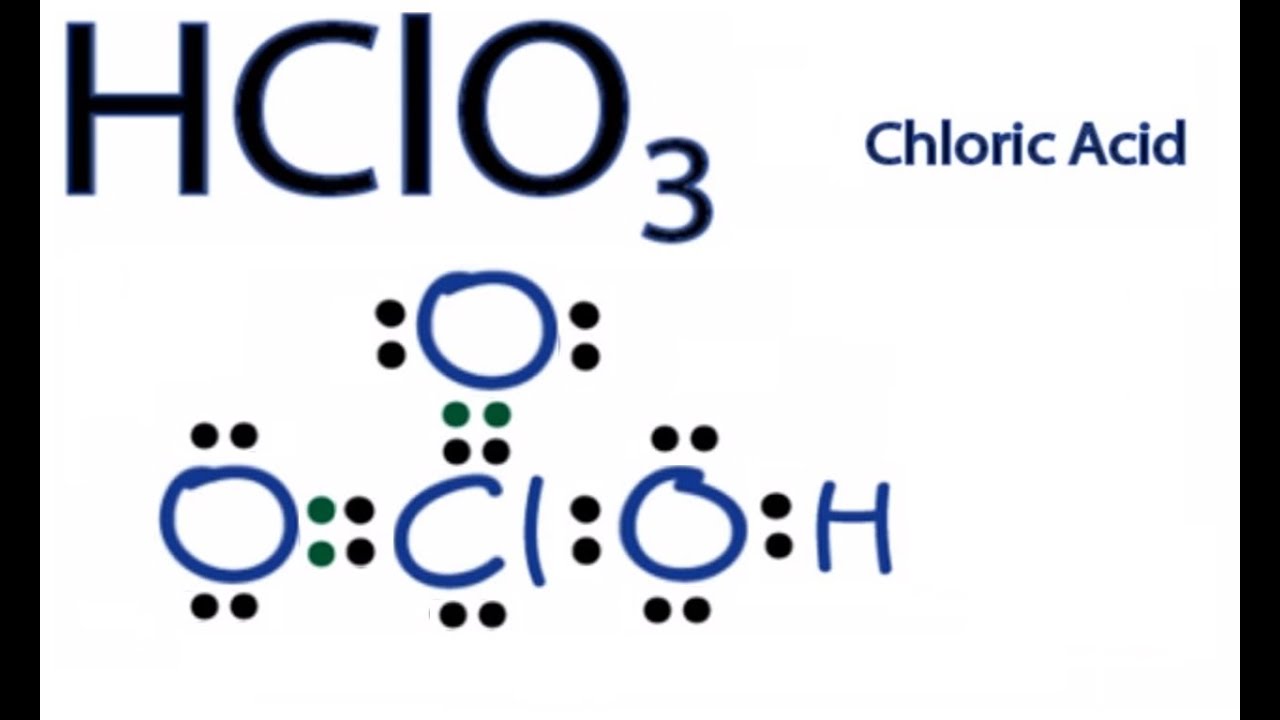
Lewis Dot Structure For Hco3. Drawing the lewis structure for hclo 3. The oxygen atom with a double bond has two lone pairs, the right oxygen atom (with which the hydrogen atom is. Determine the lewis dot structure for chloric acid:hclo3. It is commonly known as iodic acid having the chemical formula hio3.
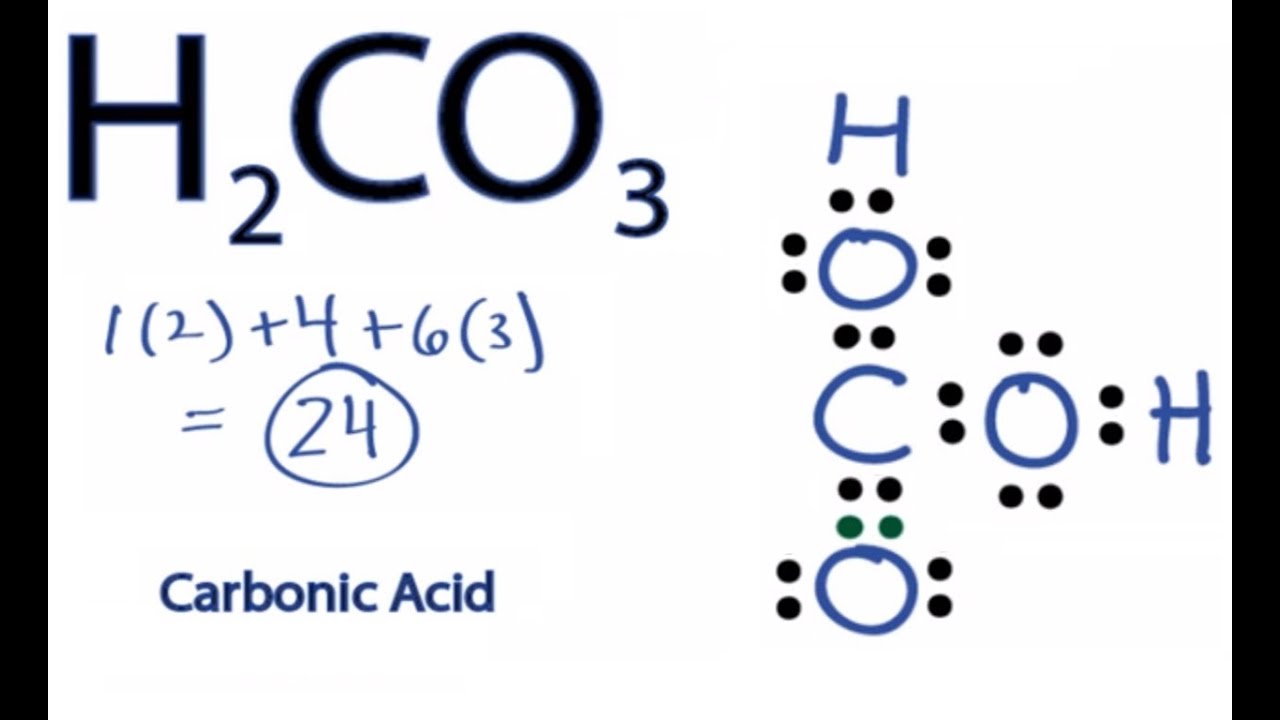 H2CO3 Lewis Structure How to Draw the Lewis Structure for Carbonic From youtube.com
H2CO3 Lewis Structure How to Draw the Lewis Structure for Carbonic From youtube.com
Resonance take place in a compound due to the tendency of extra negative ion to create pi binds by breaking another pi bond present in the compound. Drawing the lewis structure for hclo 3. Important part here also is that h likes to bond with o. The oxygen atom with a double bond has two lone pairs, the right oxygen atom (with which the hydrogen atom is. How to draw the lewis structure for h3po4. In the salt industry, it is used to synthesize various iodate like sodium, potassium iodate to increase the iodine content in the salt.
Carbonic acid is a molecule which contains one carbon atom, three oxygen atom and two hydrogen atom.
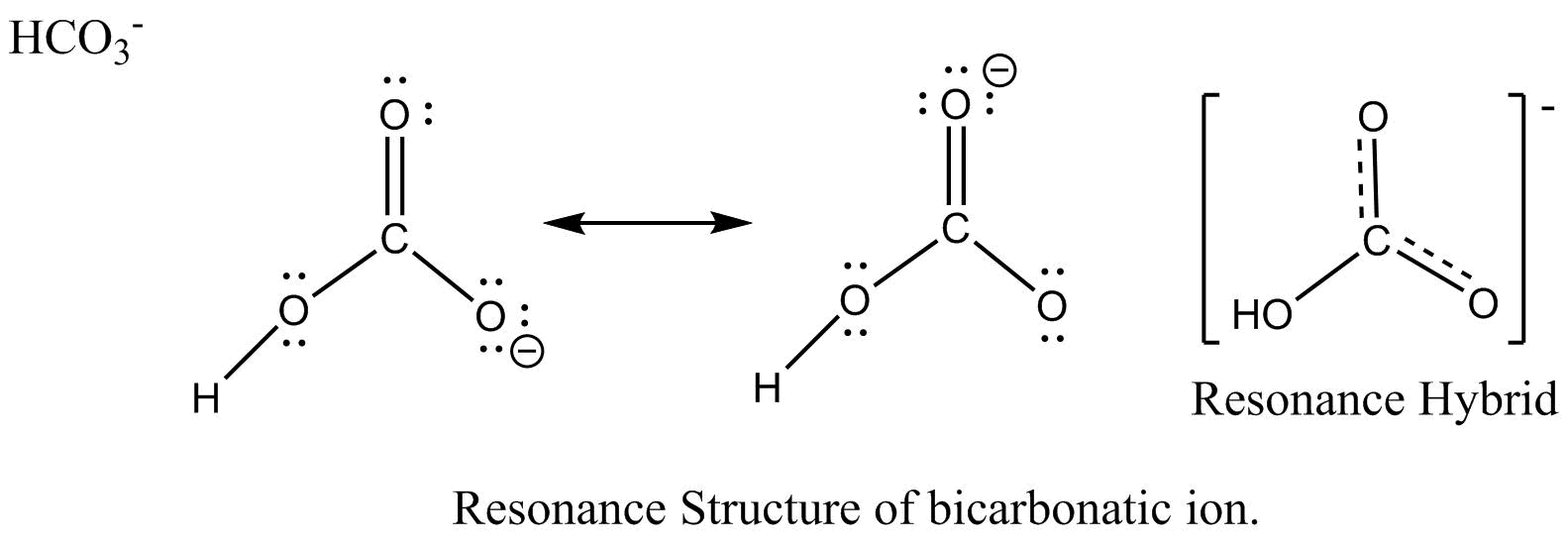
However, when carbon dioxide is water, only a little quantity of the gas is dissolved in water. Calculate the total number of valence electrons. I�m pretty sure that the bicarbonate ion, hco3 has a negative one charge. Remember, hydrogen always goes on the outside. While selecting the atom, always put the least electronegative atom at the center. The molecular mass of hio3 is 175.91 g/mol.

Remember, hydrogen always goes on the outside. H(1), c(4), o(6), if we sum it up we get 21+4+36=24. It is commonly known as iodic acid having the chemical formula hio3. Calculate the total number of valence electrons. I�m pretty sure that the bicarbonate ion, hco3 has a negative one charge.
 Source: vedantu.com
Source: vedantu.com
How to draw the lewis structure for h3po4. Lewis structure of compounds containing negative ions and pi bonds are reliable developing more than one resonating structure. The electrons are denoted by dots. Bicarbonate ion contains one carbon atom, three oxygen atoms and one hydrogen atom. I�m pretty sure that the bicarbonate ion, hco3 has a negative one charge.
 Source: youtube.com
Source: youtube.com
The molecular mass of hio3 is 175.91 g/mol. H(1), c(4), o(6), if we sum it up we get 21+4+36=24. Now for the most important rule t. It is commonly known as iodic acid having the chemical formula hio3. In the hclo 3 lewis structure chlorine is least electron electronegative atom and goes in the center of the lewis structure.
 Source: learn.lif.co.id
Source: learn.lif.co.id
Remember, hydrogen always goes on the outside. I�m pretty sure that the bicarbonate ion, hco3 has a negative one charge. Calculate the total number of valence electrons. The total valence electron is 26 for drawing the hio3 lewis structure. I�m pretty sure that the bicarbonate ion, hco3 has a negative one charge.
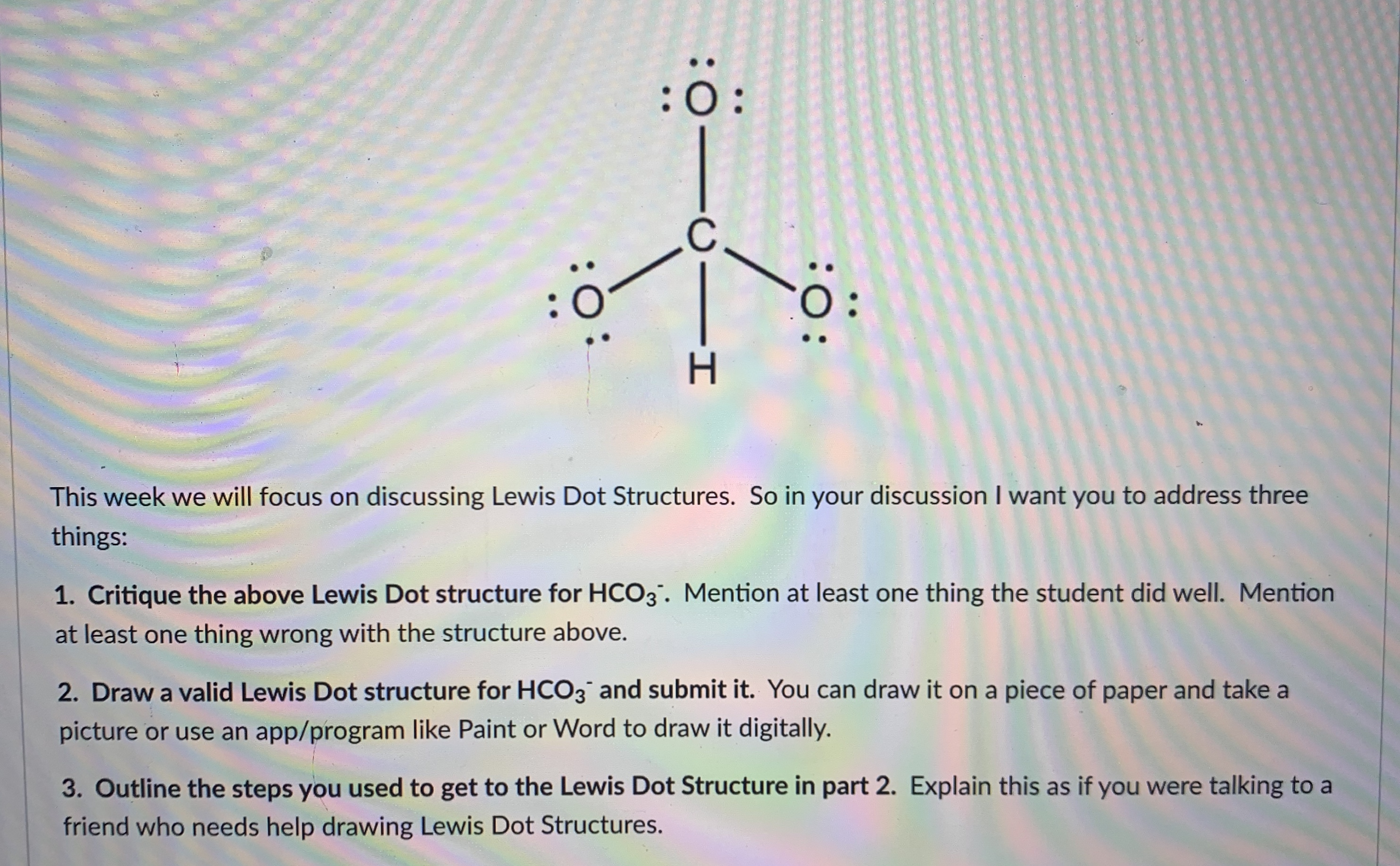 Source: bartleby.com
Source: bartleby.com
Important part here also is that h likes to bond with o. While selecting the atom, always put the least electronegative atom at the center. In the salt industry, it is used to synthesize various iodate like sodium, potassium iodate to increase the iodine content in the salt. Lewis structure of compounds containing negative ions and pi bonds are reliable developing more than one resonating structure. Now that we have the number of valence electrons we identify the central atom which is c.
 Source: clutchprep.com
Source: clutchprep.com
When we have an h (or h2) in front of a polyatomic molecule (like co 3, so 4, no 2, etc.) we know that it�s an acid.this means that the hydrogen. In the salt industry, it is used to synthesize various iodate like sodium, potassium iodate to increase the iodine content in the salt. Now that we have the number of valence electrons we identify the central atom which is c. I�m pretty sure that the bicarbonate ion, hco3 has a negative one charge. Carbonic acid is a molecule which contains one carbon atom, three oxygen atom and two hydrogen atom.
 Source: youtube.com
Source: youtube.com
Drawing the lewis structure for hclo 3. Method for drawing the lewis configuration of chloric acid, hclo3, chloric acid, hydro gen, hclo3 lewis structure, electron dot lewis structure of chloric acid, chloric acid structure, lewis dot structure of hclo3, chemistry help, online chemistry help, chemistry net, lewis electron dot structure of hclo3 chloric acid, σ bonds in hclo3 chloric acid, π bonds in. The electrons are denoted by dots. Drawing the lewis structure for hclo 3. While selecting the atom, always put the least electronegative atom at the center.
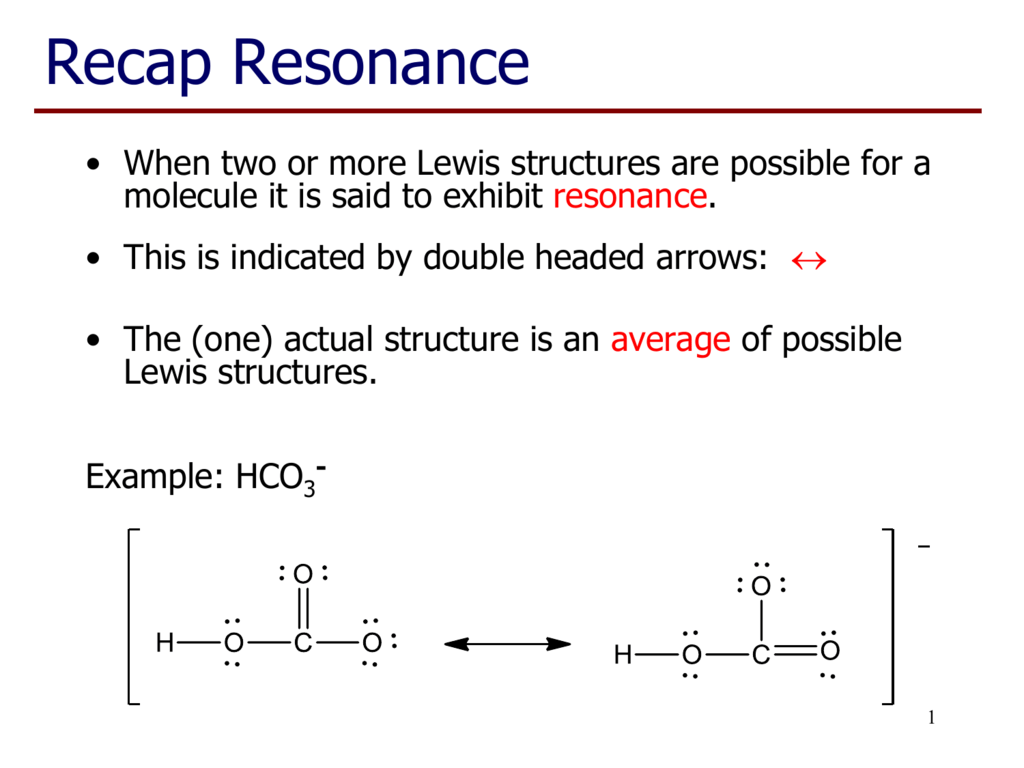 Source: learn.lif.co.id
Source: learn.lif.co.id
The total valence electron is 26 for drawing the hio3 lewis structure. In the salt industry, it is used to synthesize various iodate like sodium, potassium iodate to increase the iodine content in the salt. When we have an h (or h2) in front of a polyatomic molecule (like co 3, so 4, no 2, etc.) we know that it�s an acid.this means that the hydrogen. How to draw the lewis structure for h3po4. The electrons are denoted by dots.
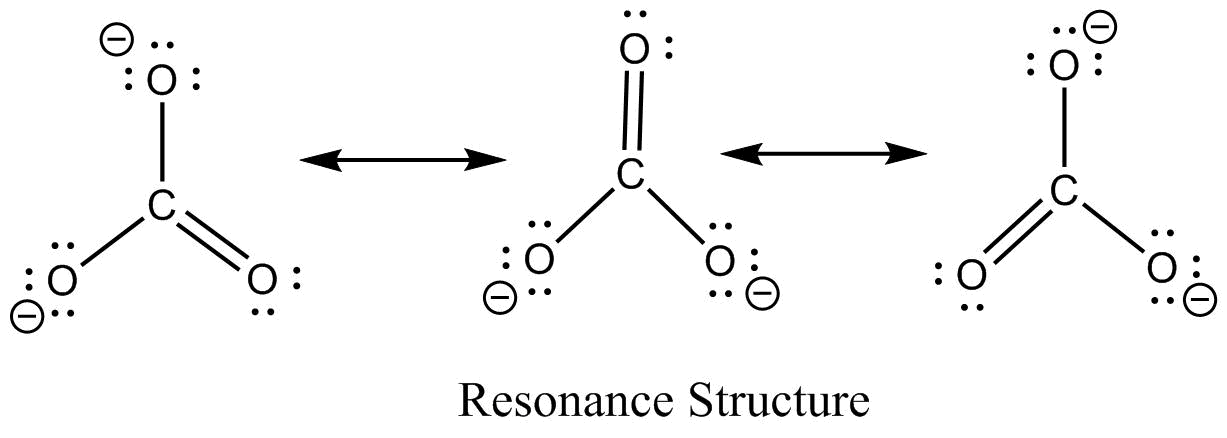 Source: vedantu.com
Source: vedantu.com
However, when carbon dioxide is water, only a little quantity of the gas is dissolved in water. I�m pretty sure that the bicarbonate ion, hco3 has a negative one charge. H(1), c(4), o(6), if we sum it up we get 21+4+36=24. In the salt industry, it is used to synthesize various iodate like sodium, potassium iodate to increase the iodine content in the salt. Bicarbonate ion contains one carbon atom, three oxygen atoms and one hydrogen atom.
 Source: youtube.com
Source: youtube.com
The total valence electron is 26 for drawing the hio3 lewis structure. The oxygen atom with a double bond has two lone pairs, the right oxygen atom (with which the hydrogen atom is. I�m pretty sure that the bicarbonate ion, hco3 has a negative one charge. While selecting the atom, always put the least electronegative atom at the center. Method for drawing the lewis configuration of chloric acid, hclo3, chloric acid, hydro gen, hclo3 lewis structure, electron dot lewis structure of chloric acid, chloric acid structure, lewis dot structure of hclo3, chemistry help, online chemistry help, chemistry net, lewis electron dot structure of hclo3 chloric acid, σ bonds in hclo3 chloric acid, π bonds in.
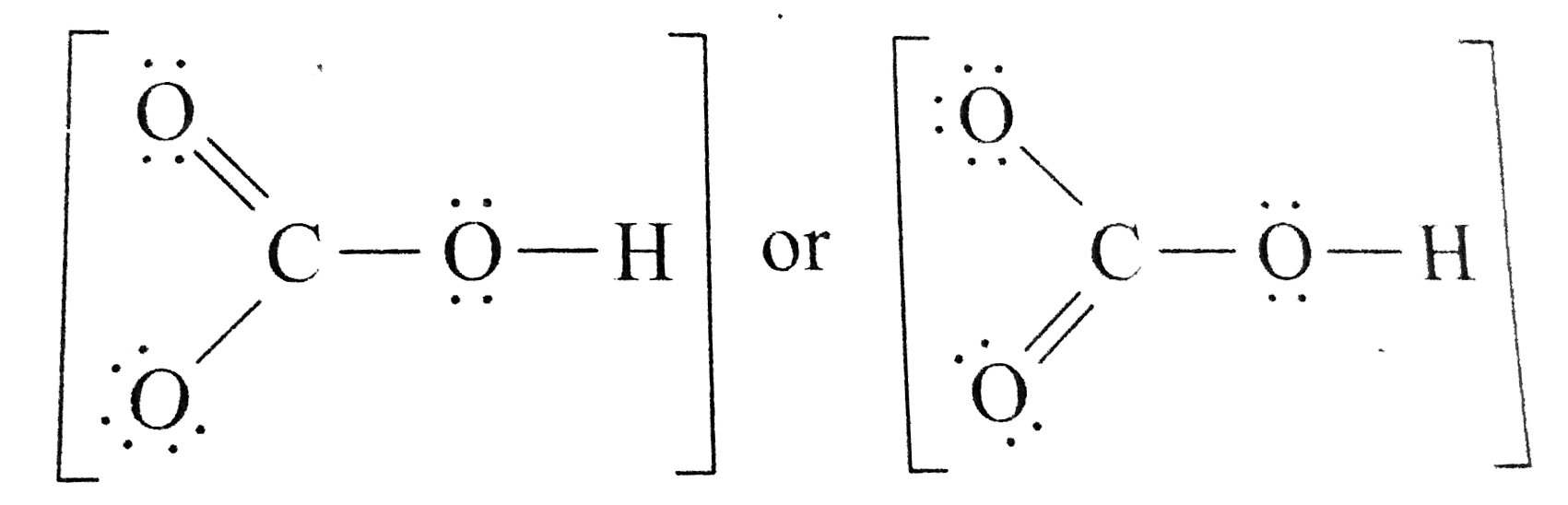
![[Solved] Write Lewis structures for these ions. (a) HCO3^ Bicarbonate ion [Solved] Write Lewis structures for these ions. (a) HCO3^ Bicarbonate ion](https://examquiz.netlify.app/img/placeholder.svg)
While selecting the atom, always put the least electronegative atom at the center. Remember, hydrogen always goes on the outside. H 2 co 3 (carbonic acid) lewis structure. Now for the most important rule t. Method for drawing the lewis configuration of chloric acid, hclo3, chloric acid, hydro gen, hclo3 lewis structure, electron dot lewis structure of chloric acid, chloric acid structure, lewis dot structure of hclo3, chemistry help, online chemistry help, chemistry net, lewis electron dot structure of hclo3 chloric acid, σ bonds in hclo3 chloric acid, π bonds in.
![[Solved] Write Lewis structures for these ions. (a) HCO3^ Bicarbonate ion [Solved] Write Lewis structures for these ions. (a) HCO3^ Bicarbonate ion](https://examquiz.netlify.app/img/placeholder.svg)
The total valence electron is 26 for drawing the hio3 lewis structure. It is a weak acid (with ph 4.18) formed when carbon dioxide (co2) dissolves in water. Carbonic acid is a molecule which contains one carbon atom, three oxygen atom and two hydrogen atom. The oxygen atom with a double bond has two lone pairs, the right oxygen atom (with which the hydrogen atom is. H2co3, known as carbonic acid is a compound of carbon, hydrogen, and oxygen.
 Source: learn.lif.co.id
Source: learn.lif.co.id
When we have an h (or h2) in front of a polyatomic molecule (like co 3, so 4, no 2, etc.) we know that it�s an acid.this means that the hydrogen. Now that we have the number of valence electrons we identify the central atom which is c. Carbonic acid is a molecule which contains one carbon atom, three oxygen atom and two hydrogen atom. Determine the lewis dot structure for chloric acid:hclo3. Now for the most important rule t.
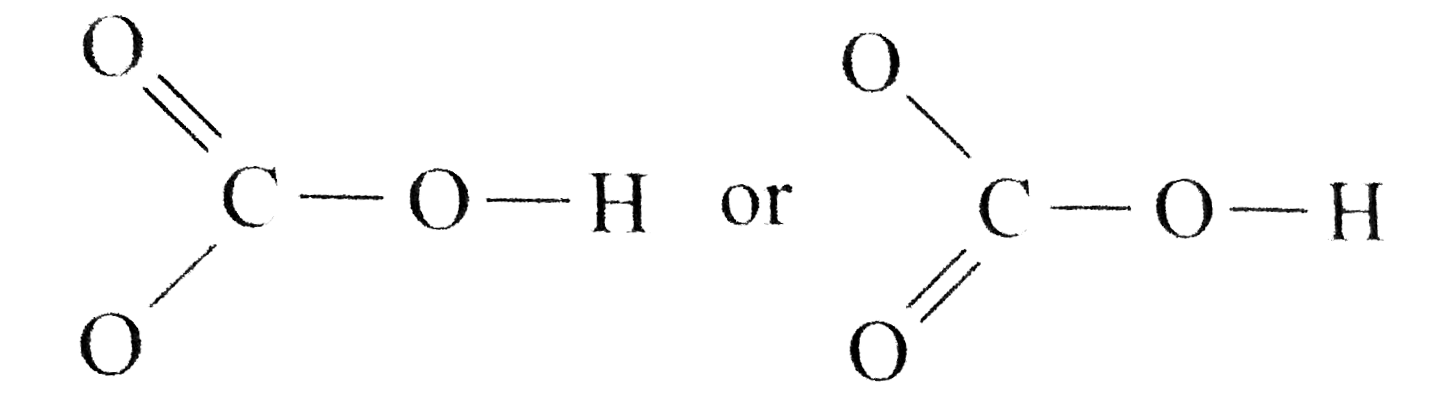 Source: learn.lif.co.id
Source: learn.lif.co.id
Calculate the total number of valence electrons. Determine the lewis dot structure for chloric acid:hclo3. Resonance take place in a compound due to the tendency of extra negative ion to create pi binds by breaking another pi bond present in the compound. H 2 co 3 (carbonic acid) lewis structure. I�m pretty sure that the bicarbonate ion, hco3 has a negative one charge.
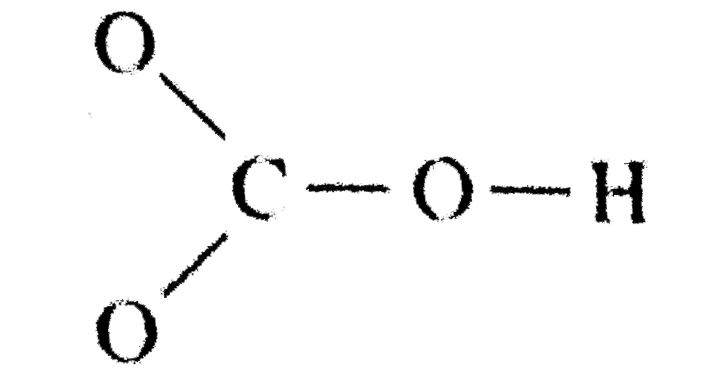 Source: learn.lif.co.id
Source: learn.lif.co.id
However, when carbon dioxide is water, only a little quantity of the gas is dissolved in water. It is a weak acid (with ph 4.18) formed when carbon dioxide (co2) dissolves in water. The oxygen atom with a double bond has two lone pairs, the right oxygen atom (with which the hydrogen atom is. Remember, hydrogen always goes on the outside. H(1), c(4), o(6), if we sum it up we get 21+4+36=24.
 Source: youtube.com
Source: youtube.com
How to draw the lewis structure for h3po4. Determine the lewis dot structure for chloric acid:hclo3. Lewis structure of compounds containing negative ions and pi bonds are reliable developing more than one resonating structure. Carbonic acid is a molecule which contains one carbon atom, three oxygen atom and two hydrogen atom. I�m pretty sure that the bicarbonate ion, hco3 has a negative one charge.
 Source: chem.libretexts.org
Source: chem.libretexts.org
It is a very unstable acid that remains in equilibrium as the solution disassociates. Carbonic acid is a molecule which contains one carbon atom, three oxygen atom and two hydrogen atom. I�m pretty sure that the bicarbonate ion, hco3 has a negative one charge. However, when carbon dioxide is water, only a little quantity of the gas is dissolved in water. H 2 co 3 (carbonic acid) lewis structure.
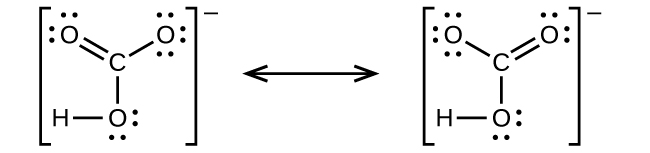 Source: opentextbc.ca
Source: opentextbc.ca
The first thing we do is count the valence electrons. Bicarbonate ion contains one carbon atom, three oxygen atoms and one hydrogen atom. The first thing we do is count the valence electrons. How to draw the lewis structure for h3po4. In the salt industry, it is used to synthesize various iodate like sodium, potassium iodate to increase the iodine content in the salt.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title lewis dot structure for hco3 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





