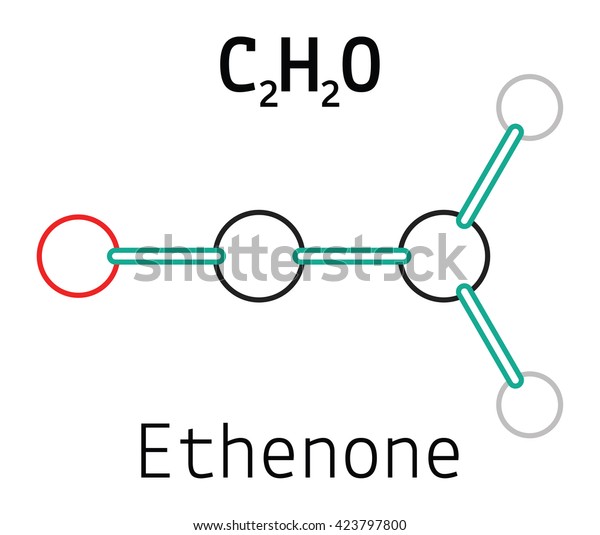
Lewis structure for c2h2o
Lewis Structure For C2h2o. In the very first step, we will count the total valence electron in the c 2 h 2 cl 2 molecule. (1) count number of valence electrons. The lewis structure of c 2 h 2 o contains one triple bond and three single bonds, with two carbons in the center. Molecular geometry of ch 2 o.
 Solved Step 3. Drag Lone Pairs Of Electrons To Each Termi… From chegg.com
Solved Step 3. Drag Lone Pairs Of Electrons To Each Termi… From chegg.com
Here’s how you can draw the c 2 h 2 lewis structure step by step. Show all unshared electron pairs. Search for how many more electrons are required to stabilize the octet of all the interacting atoms: The lewis structure of ch2o is drawn as: Drawing the lewis structure for c 2 h 2 (ethyne or acetylene). (2) put least electronegative atom in middle (but not h.
Search for how many more electrons are required to stabilize the octet of all the interacting atoms:
Thus, ch2o has a total of twelve valence electrons that can help in drawing its lewis structure. A hydrogen atom has a valency of one as it only one electron in its outer shell. Do xef4 have a lewis structure? The o atom has two. In drawing the lewis structure for c 2 h 2 also called ethyne youll find that you dont have enough valence. Count total valence electron in c 2 h 2 cl 2.
 Source: howto.lif.co.id
Source: howto.lif.co.id
In drawing the lewis structure for c 2 h 2 also called ethyne youll find that you dont have enough valence. Drawing the lewis structure for c 2 h 2 (ethyne or acetylene). Therefore, the attached image is the lewis structure of. None of the atoms bears a formal charge, and all atoms have octets (except for hydrogen atoms, which have duets). In the very first step, we will count the total valence electron in the c 2 h 2 cl 2 molecule.
 Source: shutterstock.com
Source: shutterstock.com
Drawing the lewis structure for c 2 h 2 (ethyne or acetylene). (1) count number of valence electrons. (2) put least electronegative atom in middle (but not h. The molecular geometry of ch 2 o is trigonal planar because the central carbon atom has no lone pair and is attached to the two hydrogen atoms and one oxygen atom through two single bonds and one double bond. Count total valence electron in c 2 h 2 cl 2.
 Source: japaneseclass.jp
Source: japaneseclass.jp
The chlorine atom belongs to the periodic group 7a. In drawing the lewis structure for c 2 h 2 also called ethyne youll find that you dont have enough valence. Show all unshared electron pairs. National center for biotechnology information. The o atom has two.
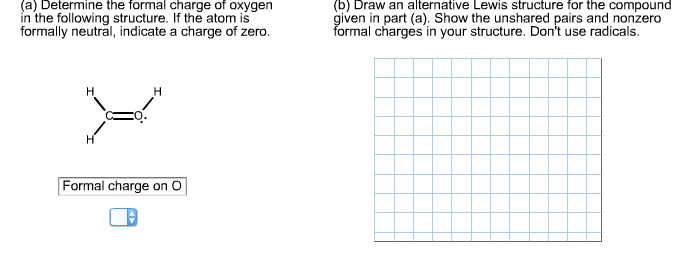 Source: chegg.com
Source: chegg.com
None of the atoms bears a formal charge, and all atoms have octets (except for hydrogen atoms, which have duets). The left carbon is attached with one hydrogen, and the right carbon is attached with oxygen and one other hydrogen. The lewis structure of ch2o is drawn as: It contains two chlorine atoms and one oxygen atom and we will learn how to draw the lewis structure of cl 2 o step by step in this tutorial. Follow some steps for drawing the lewis structure for c 2 h 2 cl 2.
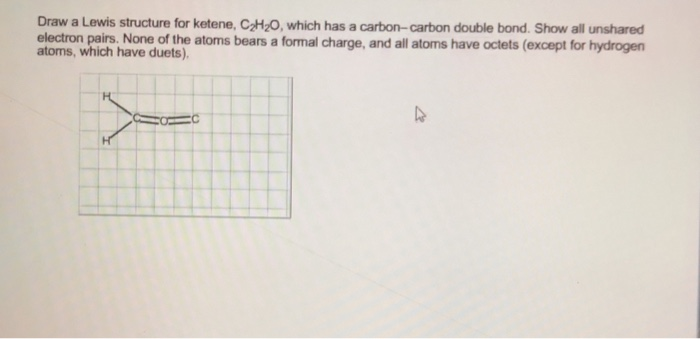 Source: draweasy9.blogspot.com
Source: draweasy9.blogspot.com
In c and o bond, the difference: It is twelve as two. In drawing the lewis structure for c 2 h 2 also called ethyne youll find that you dont have enough valence. The molecular geometry of ch 2 o is trigonal planar because the central carbon atom has no lone pair and is attached to the two hydrogen atoms and one oxygen atom through two single bonds and one double bond. Since the overall formal charge is zero, the above lewis structure of ch 2 o is most appropriate, reliable, and stable in nature.
 Source: yarravillemarket.blogspot.com
Source: yarravillemarket.blogspot.com
Show all unshared electron pairs. Advertisement advertisement new questions in chemistry. On the unbonded sides, there are five single bonded h atoms and one single bonded o atom. Molecular geometry of ch 2 o. = 12 valence electrons of ch2o.
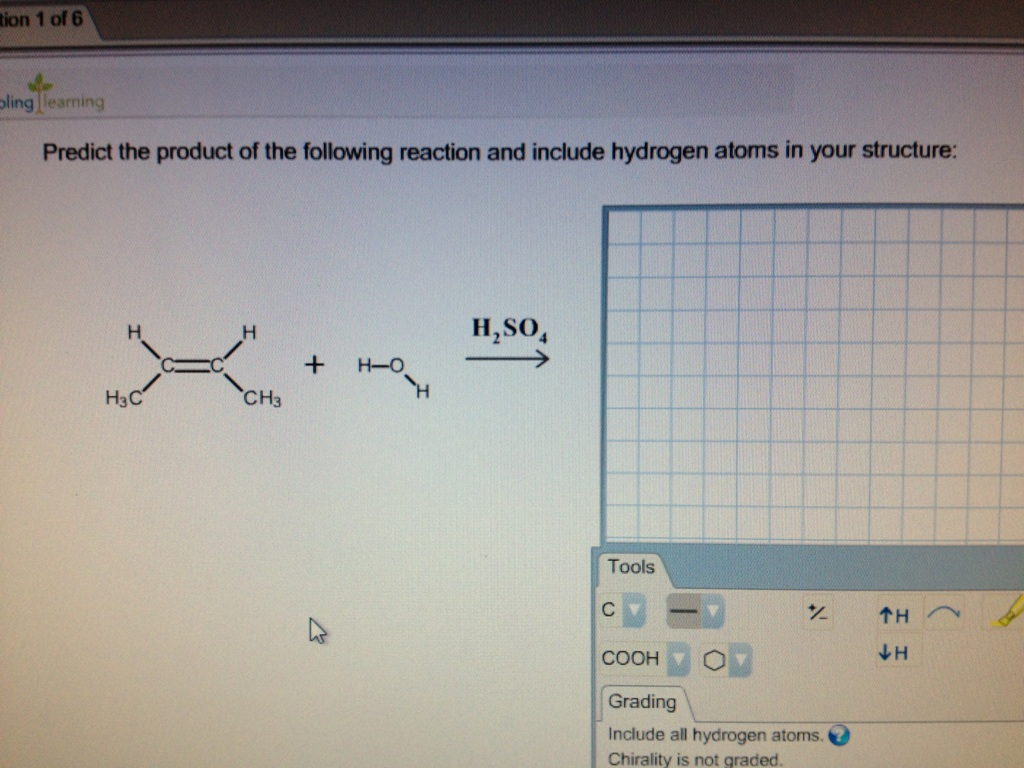 Source: chegg.com
Source: chegg.com
It is twelve as two. Thus, ch2o has a total of twelve valence electrons that can help in drawing its lewis structure. The solution is to share three pairs of valence. On the left, we have the oxygen atom between the two carbons. Total valence electron of ch2o= valence electrons of carbon + valence electrons of oxygen + valence electrons of hydrogen.
![[Solved] Answer the following The skeleton of acetic acid isshown.Draw [Solved] Answer the following The skeleton of acetic acid isshown.Draw](https://examquiz.netlify.app/img/placeholder.svg)
National center for biotechnology information. Do xef4 have a lewis structure? (1) count number of valence electrons. Both use all 20 valence e. Thus, ch2o has a total of twelve valence electrons that can help in drawing its lewis structure.
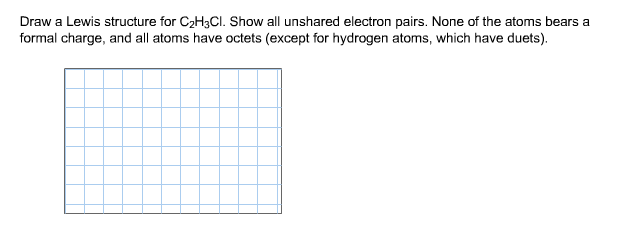 Source: chegg.com
Source: chegg.com
On the left, we have the oxygen atom between the two carbons. Follow some steps for drawing the lewis structure for c 2 h 2 cl 2. Here, we have a diagram of pauling electronegativity chart: This is called dimethyl ether. It is widely used as a preservative because of its antibacterial.
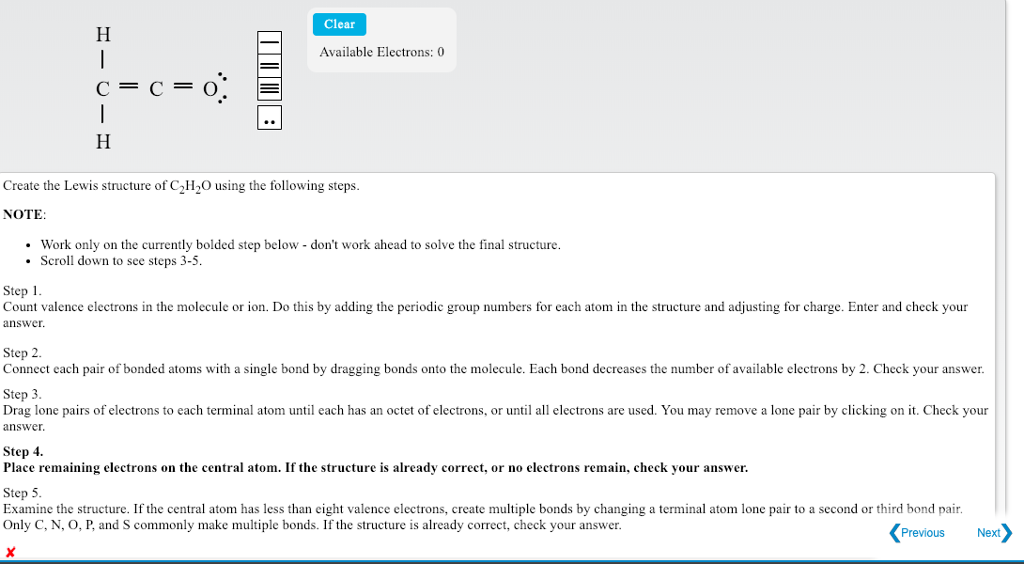 Source: chegg.com
Source: chegg.com
Follow some steps for drawing the lewis structure for c 2 h 2 cl 2. The lewis (dot) structure for h2co (methanal or formaldehyde) is: The lewis structure of ch2o is drawn as: Drawing the lewis structure for c 2 h 2 (ethyne or acetylene). In c and o bond, the difference:
 Source: chegg.com
Source: chegg.com
Formaldehyde is an organic compound that appears as a colorless gas with the chemical formula ch2o. So there are two ways presented here to draw the c2h6o lewis structure. In drawing the lewis structure for c 2 h 2 also called ethyne youll find that you dont have enough valence. The chlorine atom belongs to the periodic group 7a. On the unbonded sides, there are five single bonded h atoms and one single bonded o atom.
 Source: pinterest.com
Source: pinterest.com
There are 5 steps for writing dot structures: Search for the total already available valence electrons in a single formaldehyde ch2o molecule: National center for biotechnology information. Just as the lewis dot structure can visualize molecules, it can also visualize polyatomic ions, which are. Formaldehyde is an organic compound that appears as a colorless gas with the chemical formula ch2o.
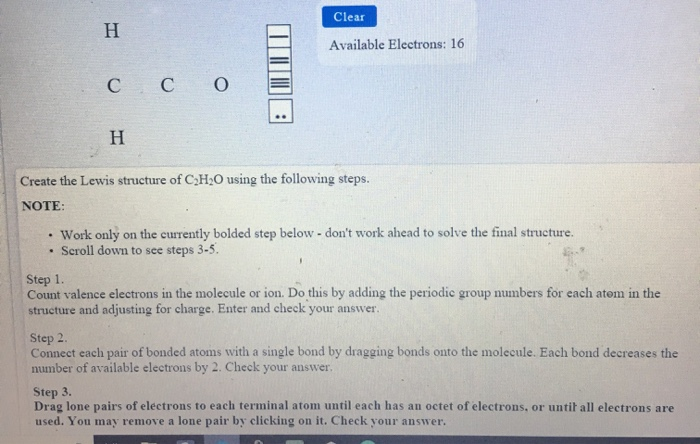 Source: draweasy9.blogspot.com
Source: draweasy9.blogspot.com
The lewis structure of c 2 h 2 o contains one triple bond and three single bonds, with two carbons in the center. It is the simplest aldehyde made up of two hydrogens, one carbon, and one oxygen. Put two electrons between the atoms to represent a chemical bond. Do xef4 have a lewis structure? Both use all 20 valence e.
 Source: chegg.com
Source: chegg.com
Cl 2 o (dichlorine monoxide) lewis structure and steps of drawing. Total is 12 as there are 2 hydrogen atoms. These pairs of electrons present between the carbon & oxygen atoms as well as between. None of the atoms bears a formal charge, and all atoms have octets (except for hydrogen atoms, which have duets). Molecular geometry of ch 2 o.
 Source: mctcteach.org
Source: mctcteach.org
Therefore, the attached image is the lewis structure of. There are 5 steps for writing dot structures: Here, we have a diagram of pauling electronegativity chart: In drawing the lewis structure for c 2 h 2 (also called ethyne) you�ll find that you don�t have enough valence electrons available to satisfy the octet for each element (if you use only single bonds). Cl 2 o (dichlorine monoxide) lewis structure and steps of drawing.
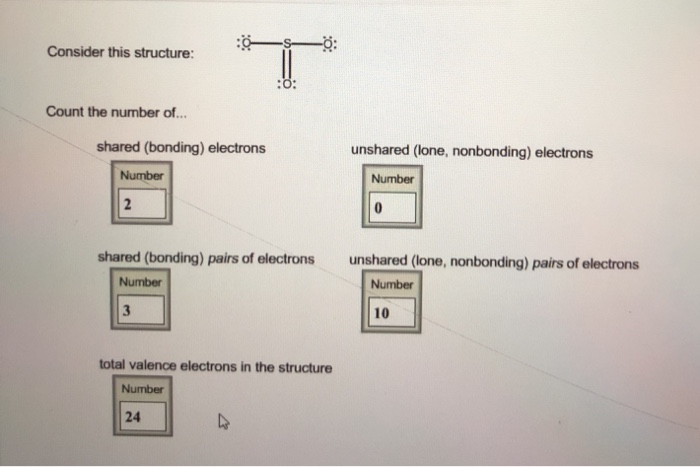 Source: chegg.com
Source: chegg.com
A hydrogen atom has a valency of one as it only one electron in its outer shell. It is twelve as two. The solution is to share three pairs of valence. Therefore, the attached image is the lewis structure of. Cl 2 o (dichlorine monoxide) lewis structure and steps of drawing.
 Source: yarravillemarket.blogspot.com
Source: yarravillemarket.blogspot.com
Search for the total already available valence electrons in a single formaldehyde ch2o molecule: Therefore, the attached image is the lewis structure of. There are 5 steps for writing dot structures: Oxygen atom is the center atom and both chlorine. It is widely used as a preservative because of its antibacterial.
 Source: jalishamav.blogspot.com
Source: jalishamav.blogspot.com
Put two electrons between the atoms to represent a chemical bond. Both use all 20 valence e. Just as the lewis dot structure can visualize molecules, it can also visualize polyatomic ions, which are. Count total valence electron in c 2 h 2 cl 2. Minimize charges again (if there are) let’s break down each step in detail.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title lewis structure for c2h2o by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.