Percent ionic character of tio2
Percent Ionic Character Of Tio2. What is an ionic compound so2 clo2 h2o2 tio2? The electronegativity difference serves as a measure of percentage at which a bond is ionic.roughly speaking, electro negativity difference of 1.7 is equivalent to 50 ℅ ionic character;.(calculated ionic character in your question ) thus, ionic character of a given compound is 50% ×∆ (e.n)/1.7 In this way unique techniques for mix of ag nanoparticles in perspective of the physical approach have been delivered and in the combination of titanium. The electronegativities of the elements are found in figure 2.7.

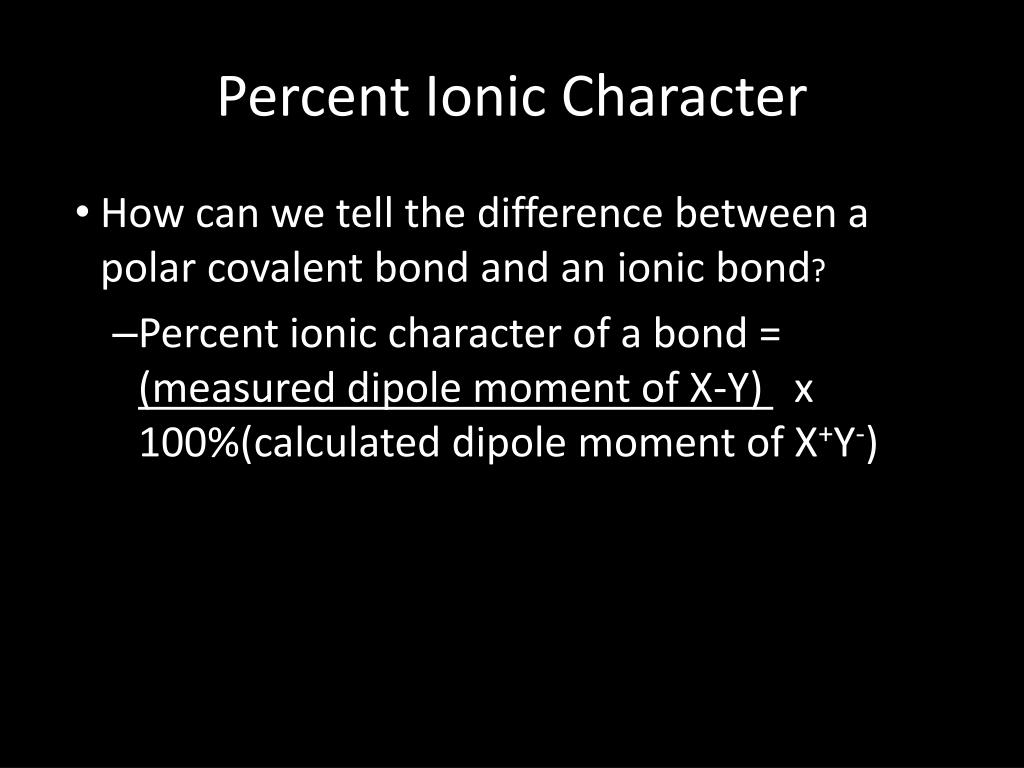
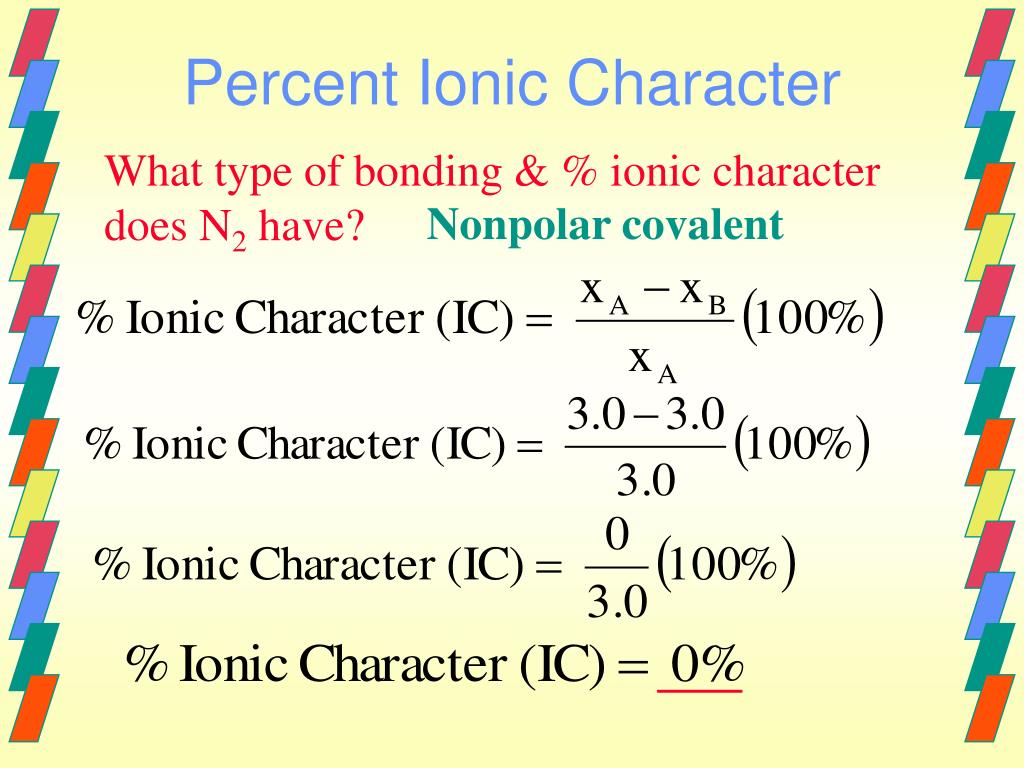
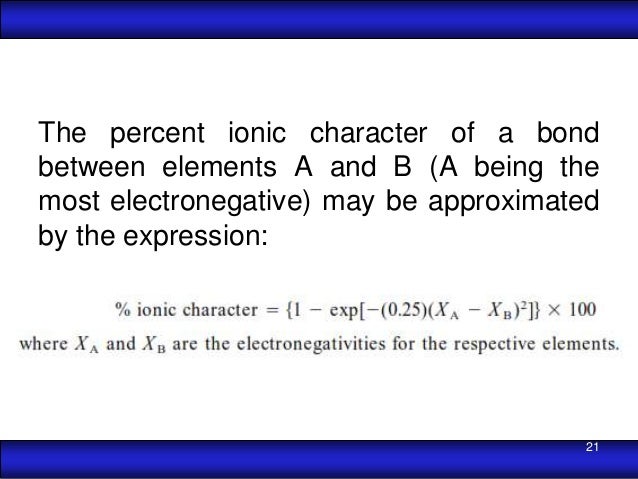
The percent ionic character is a function of the electro negativities of the ions xa and xb according to below equation : Tio2, znte, cscl, insb, and mgcl2. The density and atomic weight for fe are 7.65 g/cm3 and 55.85 g/mol, respectively 3. The electronegativity values of cs and cl are 0.7 and 0.3 respectively. I�m not sure of the % ionic charachter, i�ve just been taught it is either ionic or covalent. The formula for percentage of nick i only directories director is one minus eateries but 14 into.
The cutoff point is 1.7, with greater than a 1.7 difference is ionic, lowe.
The electronegativity values of cs and cl are 0.7 and 0.3 respectively. The cutoff point is 1.7, with greater than a 1.7 difference is ionic, lowe. In this problem we have to compute the ionic director percentages of intra atomic bones for the following compounds. Offer an explanation as to. Calculate the number of vacancies per cubic meter in iron at 850°c. When the difference in electronegativities is great, the orbital may be so far over to one side that it barely.
 Source: chegg.com
Source: chegg.com
Tio2, znte, cscl,insb, and mgcl2. Using the difference of ionic electronegativity expressed/designated as xa and xb. Priyavaghela7743 priyavaghela7743 15.09.2020 chemistry secondary school answered The percent ionic character is a function of the electron negativities of the ions x a and x b according to equation 210. Mixed bond (not ionic, not covalent).

In this problem we have to compute the ionic director percentages of intra atomic bones for the following compounds. The electronegativity values of cs and cl are 0.7 and 0.3 respectively. The density and atomic weight for fe are 7.65 g/cm3 and 55.85 g/mol, respectively 3. Tio2, znte, cscl,insb, and mgcl2. In this way unique techniques for mix of ag nanoparticles in perspective of the physical approach have been delivered and in the combination of titanium.
 Source: chegg.com
Source: chegg.com
The percent ionic character is a function of the electro negativities of the ions xa and xb according to below equation : In this problem we have to compute the ionic director percentages of intra atomic bones for the following compounds. What is an ionic compound so2 clo2 h2o2 tio2? The bonding is predominantly ionic (but with some covalent character) on the basis of the relative positions of. Compute the ionic character percentages of the interatomic bonds for the following compounds:
 Source: chegg.com
Source: chegg.com
The percent ionic character is a function of the electron negativities of the ions x a and x b according to equation 210. The density and atomic weight for fe are 7.65 g/cm3 and 55.85 g/mol, respectively 3. In this way unique techniques for mix of ag nanoparticles in perspective of the physical approach have been delivered and in the combination of titanium. The energy for vacancy formation is 1.08 ev/atom. I�m not sure of the % ionic charachter, i�ve just been taught it is either ionic or covalent.
 Source: slideserve.com
Source: slideserve.com
Priyavaghela7743 priyavaghela7743 15.09.2020 chemistry secondary school answered Compute the percents ionic character of the interatomic bonds for each of the following compounds: Compute the percent ionic character of the interatomic bonds for the following compounds : It all revolves around the difference in electronegtivity between the two elements or radicals in the compound. In this problem we have to compute the ionic director percentages of intra atomic bones for the following compounds.
 Source: oneclass.com
Source: oneclass.com
Tio2, znte, cscl, insb, and mgcl2. Find an answer to your question compute the percent ionic character of the interatomic bonds for the following compound: Tio2, znte, csci, insb, and mgcl2. Tio2, znte, cscl, insb, and mgcl2. Percent ionic character of tio2.
 Source: chegg.com
Source: chegg.com
Answer to compute the percents ionic character of the interatomic bonds for the following compounds: The percent ionic character is a function of the electro negativities of the ions xa and xb according to below equation : The procedure is put in your electronegativities, (the order doesn�t matter because we square the difference) and then square the difference. Compute the percents ionic character of the interatomic bonds for each of the following compounds: Tio2, znte, cscl, insb, and mgcl2.
 Source: chegg.com
Source: chegg.com
Tio2 is ionic, rest are covalent compounds. This problem has been solved! The electronegativities of the elements are found in figure 2.7. Tio2, znte, cscl,insb, and mgcl2. Tio2, znte, cscl, insb, and mgcl2.
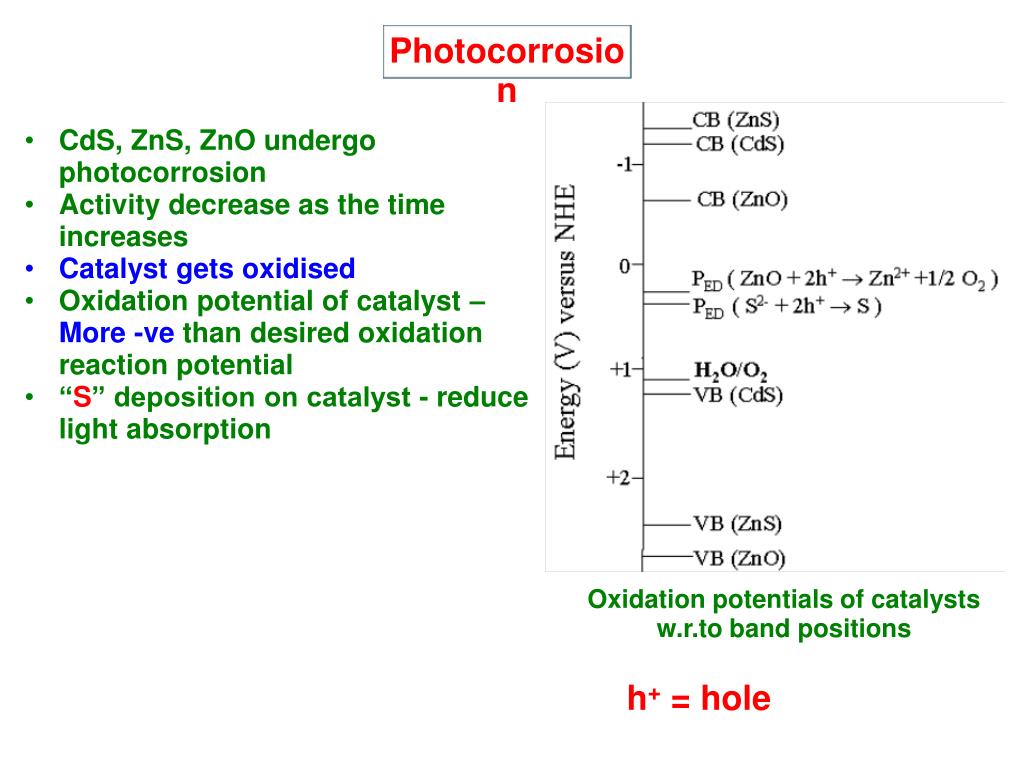 Source: slideserve.com
Source: slideserve.com
The electronegativity difference serves as a measure of percentage at which a bond is ionic.roughly speaking, electro negativity difference of 1.7 is equivalent to 50 ℅ ionic character;.(calculated ionic character in your question ) thus, ionic character of a given compound is 50% ×∆ (e.n)/1.7 When the difference in electronegativities is great, the orbital may be so far over to one side that it barely. Calculate the number of vacancies per cubic meter in iron at 850°c. Compute the percents ionic character of the interatomic bonds for each of the following compounds: Hansajithnimesh4 hansajithnimesh4 09.04.2021 chemistry secondary school answered.
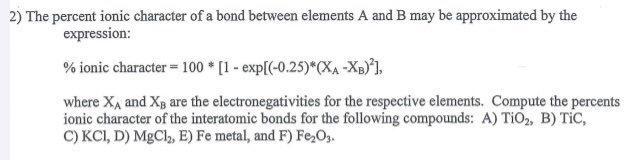 Source: chegg.com
Source: chegg.com
The cutoff point is 1.7, with greater than a 1.7 difference is ionic, lowe. Answer of compute the percents ionic character of the interatomic bonds for the following compounds: Answer to compute the percents ionic character of the interatomic bonds for the following compounds: Tio2, znte, cscl,insb, and mgcl2. Tio2, znte, cscl, insb, and mgcl2.

Compute the percent ionic character of the interatomic bonds for each of the following compounds: Tio2, znte, cscl, insb, and mgcl2. I�m not sure of the % ionic charachter, i�ve just been taught it is either ionic or covalent. When the difference in electronegativities is great, the orbital may be so far over to one side that it barely. Percent ionic character of tio2.
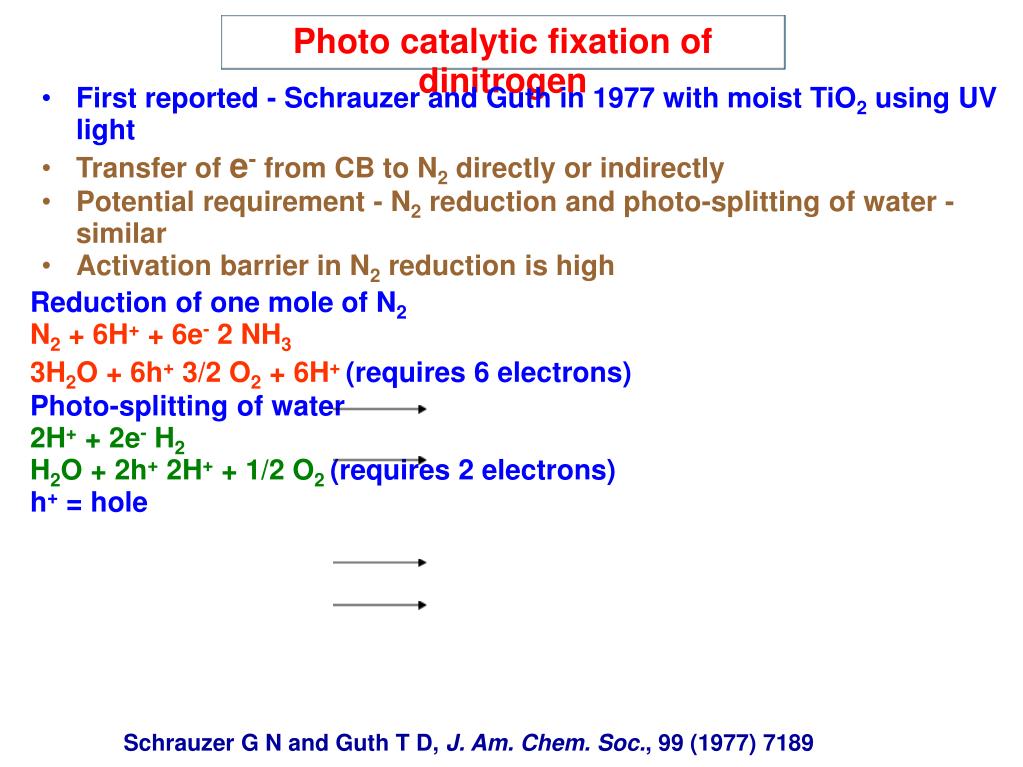 Source: slideserve.com
Source: slideserve.com
2.19 the percent ionic character is a function of the electron. Tio2 is ionic, rest are covalent compounds. Tio2, znte, csci, insb, and mgcl2. Compute the percent ionic character of the interatomic bonds for each of the following compounds: The density and atomic weight for fe are 7.65 g/cm3 and 55.85 g/mol, respectively 3.
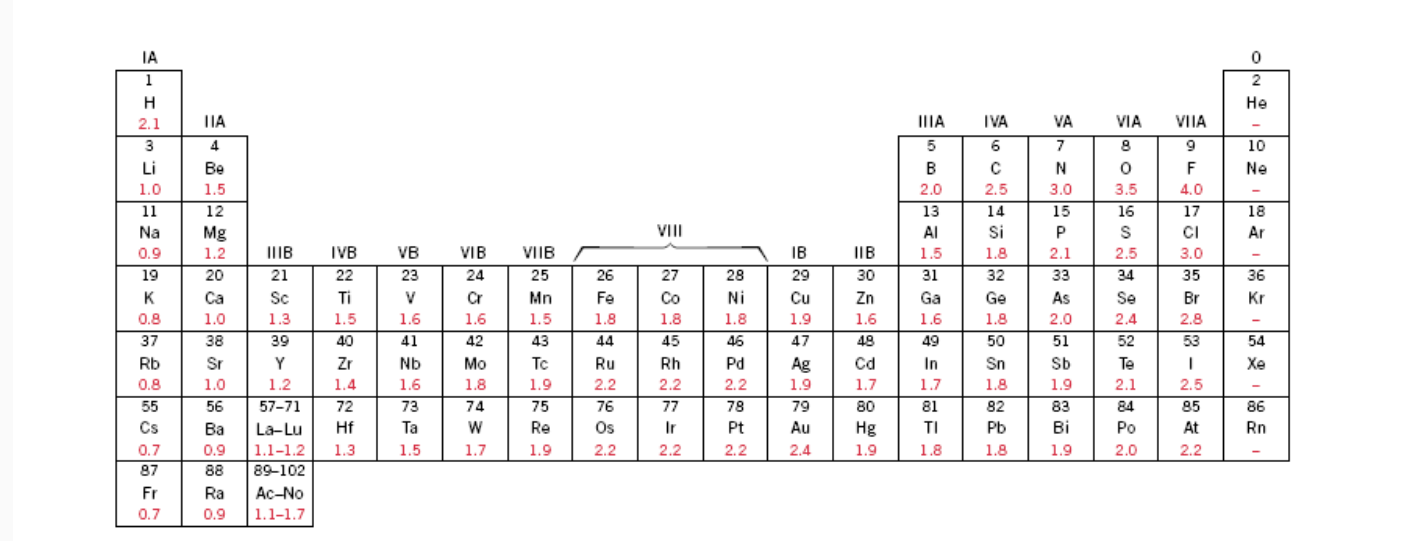
The bonding is predominantly ionic (but with some covalent character) on the basis of the relative positions of. Tio2 is ionic, rest are covalent compounds. Using the difference of ionic electronegativity expressed/designated as xa and xb. Tio2, znte, cscl,insb, and mgcl2. The procedure is put in your electronegativities, (the order doesn�t matter because we square the difference) and then square the difference.
 Source: researchgate.net
Source: researchgate.net
Tio2, znte, csci, insb, and mgcl2. This problem has been solved! The density and atomic weight for fe are 7.65 g/cm3 and 55.85 g/mol, respectively 3. Tio2, znte, cscl, insb, and mgcl2. The electronegativities of the elements are found in figure 2.7.

The electronegativity values of cs and cl are 0.7 and 0.3 respectively. Answer of compute the percents ionic character of the interatomic bonds for the following compounds: When the difference in electronegativities is great, the orbital may be so far over to one side that it barely. The cutoff point is 1.7, with greater than a 1.7 difference is ionic, lowe. It all revolves around the difference in electronegtivity between the two elements or radicals in the compound.
 Source: slideserve.com
Source: slideserve.com
The energy for vacancy formation is 1.08 ev/atom. Tio2, znte, cscl,insb, and mgcl2. The bonding is predominantly ionic (but with some covalent character) on the basis of the relative positions of. Compute the percent ionic character of the interatomic bonds for each of the following compounds: The electronegativity values of cs and cl are 0.7 and 0.3 respectively.
 Source: chegg.com
Source: chegg.com
The procedure is put in your electronegativities, (the order doesn�t matter because we square the difference) and then square the difference. Percent ionic character of tio2. Tio2 is ionic, rest are covalent compounds. Percent ionic character of tio2. Tio2, znte, cscl, insb, and mgcl2.
 Source: es.slideshare.net
Source: es.slideshare.net
Offer an explanation as to. In this way unique techniques for mix of ag nanoparticles in perspective of the physical approach have been delivered and in the combination of titanium. Tio2, znte, cscl, insb, and mgcl2. What is an ionic compound so2 clo2 h2o2 tio2? 2.19 the percent ionic character is a function of the electron.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title percent ionic character of tio2 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.