What are the 7 properties of water
What Are The 7 Properties Of Water. For example, seawater freezes at about −2 °c (28 °f). Thus, it provides a suitable medium for chemical reactions as acts as a good ionizer. This supports life because this is how organisms absorb nutrients. But below 4°c water become less dense because they placed apart because of it so ice is less dense than liquid water and floats on surface water surface ice insulated the underwater film freezing and protect the aquatic life.
 Water’s life supporting properties From slideshare.net
Water’s life supporting properties From slideshare.net
Solid (ice), liquid (water), and gas (steam). According to coulomb�s law the force between two charged particles is given by. Water is a colourless, odourless and tasteless liquid in its natural state. There are three different forms of water, or h 2 o: The less electronegative one (hydrogen) becomes partially positively charged. Ice is less dense than liquid water.
H 2 o is the chemical formula for water.
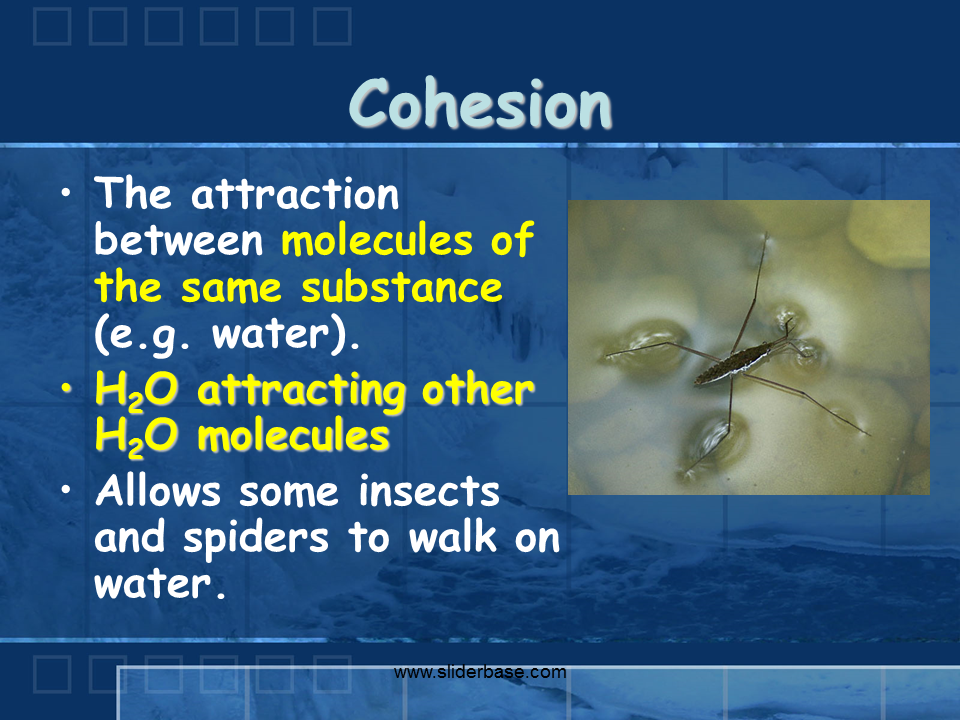
These partial charges cause the attraction of the molecules to form hydrogen bonds. Start studying 7 properties of water. Water conducts heat more easily than any liquid except mercury. Water has lower density on freezing: Some physical properties are listed below. These partial charges cause the attraction of the molecules to form hydrogen bonds.

Surface tension, heat of vaporization, and vapor pressure. These partial charges cause the attraction of the molecules to form hydrogen bonds. There are three different forms of water, or h 2 o: Water is a stable compound and does not decompose easily. Water can dissolve many ionic and polar molecules.
 Source: slideshare.net
Source: slideshare.net
Click card to see definition 👆. H 2 o is the chemical formula for water. Ice is less dense than liquid water. It is a universal solvent. The following are basic characteristics of water including physical, chemical and biological properties.

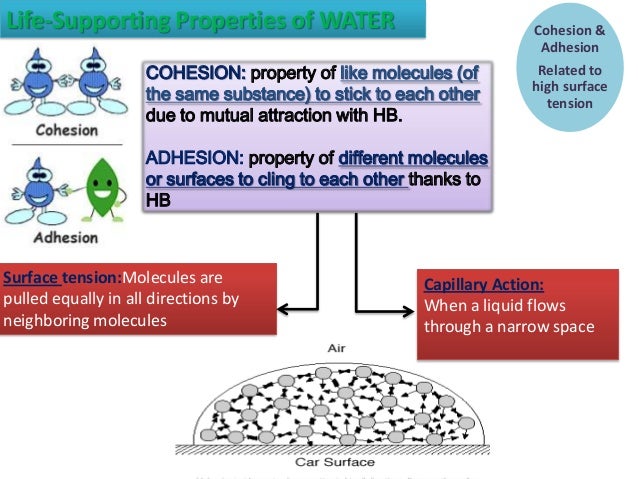
Occurring at or below 0 o c water becomes a solid crystalline form or phase. Water in a pure state has a neutral ph. Because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including: Here you need to realize water is adhesive to any molecule and form hydrogen bonds with it. Water attracting to other surfaces.
 Source: slideshare.net
Source: slideshare.net
Learn vocabulary, terms, and more with flashcards, games, and other study tools. Click card to see definition 👆. Surface tension, heat of vaporization, and vapor pressure. Life also exists approximately in this range of temperature. Water has a high specific heat.
 Source: pt.slideshare.net
Source: pt.slideshare.net
What are the 7 properties of water and its importance? H 2 o is the chemical formula for water. Thus, it provides a suitable medium for chemical reactions as acts as a good ionizer. Water can dissolve many ionic and polar molecules. As a result, the molecule is slightly charged at the ends.
 Source: cursa.ihmc.us
Water gives protection to the organisms from thermal shock. Ice is less dense than. Water can dissolve many ionic and polar molecules. The less electronegative one (hydrogen) becomes partially positively charged. Start studying 7 properties of water.
 Source: slideshare.net
Source: slideshare.net
According to coulomb�s law the force between two charged particles is given by. As a result, the molecule is slightly charged at the ends. Ice is less dense than. What are the 7 properties of water and its importance? Ice is less dense than liquid water.
 Source: studylib.net
Source: studylib.net
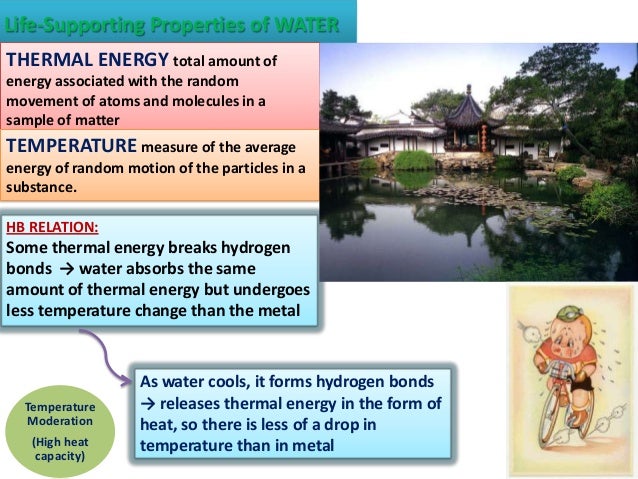
Water has a high specific heat. 0 °c (32 °f) at 1 atmosphere of pressure. Life also exists approximately in this range of temperature. Liquid water is a universal solvent. Because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including:
 Source: slideshare.net
Source: slideshare.net
Because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including: The physical properties of water are directly concerned with the appearance and interaction of water. Water is liquid between 4 and 90°c. The following are basic characteristics of water including physical, chemical and biological properties. Water is a colourless, odourless and tasteless liquid in its natural state.

The density of water is about 1 gm/cc and it varies with temperature in an undefined pattern. For example, seawater freezes at about −2 °c (28 °f). Start studying 7 properties of water. Click again to see term 👆. Water gives protection to the organisms from thermal shock.
Life also exists approximately in this range of temperature. Click card to see definition 👆. Water is liquid between 4 and 90°c. Water gives protection to the organisms from thermal shock. These partial charges cause the attraction of the molecules to form hydrogen bonds.
 Source: slideshare.net
Source: slideshare.net
Ice is less dense than. Water in a pure state has a neutral ph. (7.2.3) f = q 1 q 2 4 π ϵ 0 r 2. Water attracting to other surfaces. The following are basic characteristics of water including physical, chemical and biological properties.
 Source: slideshare.net
Source: slideshare.net
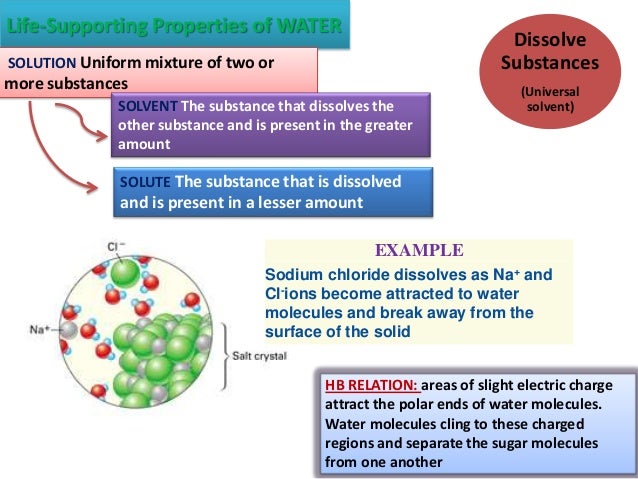
Some physical properties are listed below. Polarity,ph of water,cohesion,adhesion,low density of ice,high heat of vaporization,high specific heat. Water is a universal solvent because most substances dissolve in it. Water is a colourless and transparent chemical substance that is the primary constituent of the earth’s crust’s streams, oceans, and lakes.it is an important fluid that plays an important role in the survival of life on earth. What are the 7 properties of water and its importance?
 Source: slideshare.net
Source: slideshare.net
The following are basic characteristics of water including physical, chemical and biological properties. Liquid water is a universal solvent. Polarity,ph of water,cohesion,adhesion,low density of ice,high heat of vaporization,high specific heat. Water creeping up thin tubes. Minerals and pressure can reduce this significantly.
 Source: slideshare.net
Source: slideshare.net
Click card to see definition 👆. Some physical properties are listed below. Water gives protection to the organisms from thermal shock. Tap card to see definition 👆. As a result, the molecule is slightly charged at the ends.
 Source: edu.glogster.com
Source: edu.glogster.com
It consists of two hydrogen atoms and one oxygen atom held together by covalent bonds. Water conducts heat more easily than any liquid except mercury. Water is the only substance that naturally occurs on earth in three distinct physical states.solid liquid and gas. Ice is less dense than. The three properties of water.
 Source: slideshare.net
Source: slideshare.net
Solid (ice), liquid (water), and gas (steam). (7.2.3) f = q 1 q 2 4 π ϵ 0 r 2. Click card to see definition 👆. Thus, it provides a suitable medium for chemical reactions as acts as a good ionizer. Water has a high specific heat.

Start studying 7 properties of water. Water has a high specific heat. There are three different forms of water, or h 2 o: In this form it occupies a definite volume. As a result, the molecule is slightly charged at the ends.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title what are the 7 properties of water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.