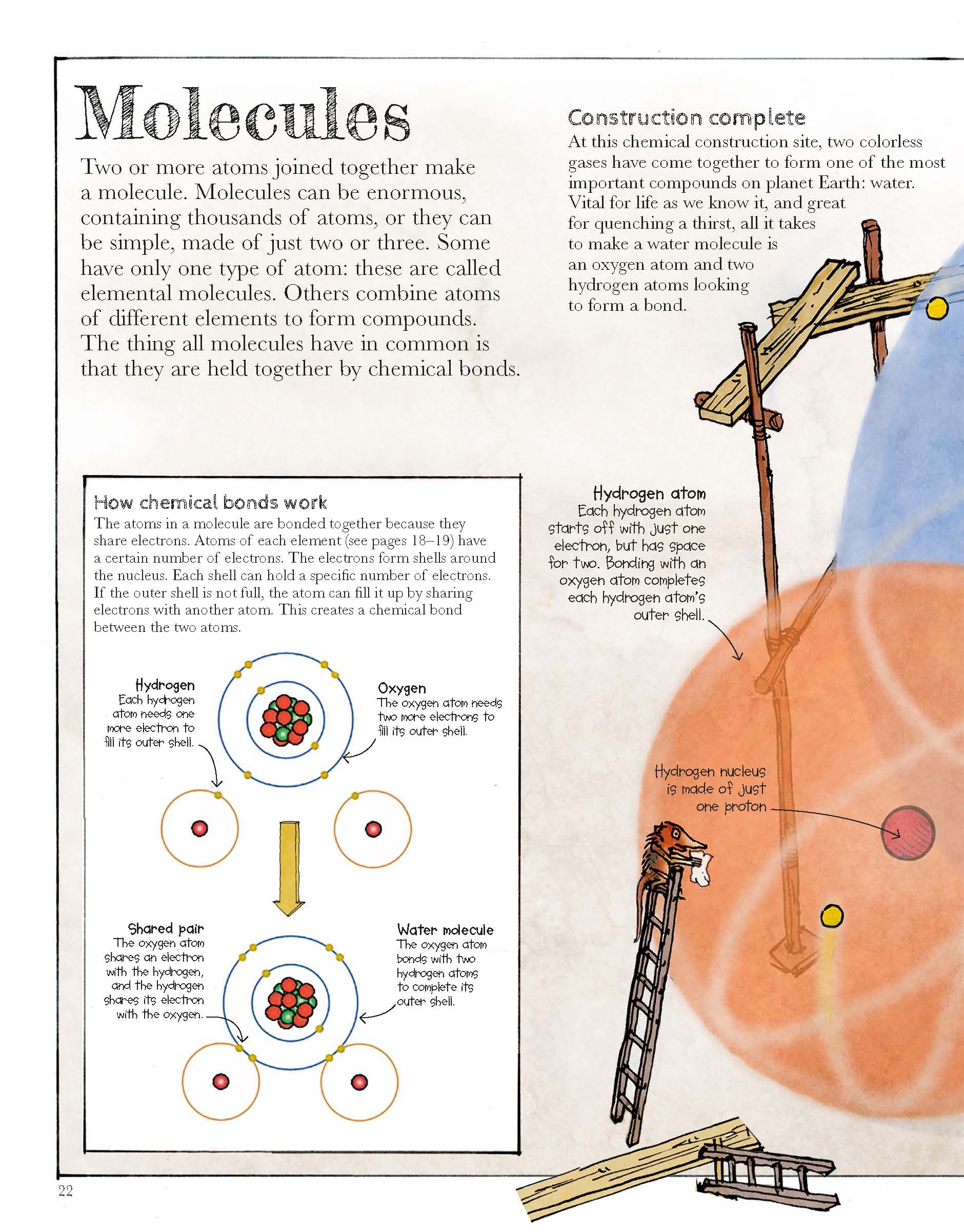
What happens when mercury oxide is heated
What Happens When Mercury Oxide Is Heated. What happens to mercury oxide is heated? What happens to the body when mercury is heated? It has a red or orange color. A reaction is also considered to be a decomposition reaction even when one or more of the.
 science chemical reaction mercuric oxide Fundamental From fphoto.photoshelter.com
science chemical reaction mercuric oxide Fundamental From fphoto.photoshelter.com
What happens when mercuric oxide is heated? What happens to mercury (ii) oxide when heated? Heating produces highly toxic mercury fumes and oxygen, which increases the fire hazard. Mercury(ii) oxide, a red solid, decomposes when heated to produce mercury and oxygen gas. When it is heated, it decomposes into mercury metal and oxygen gas. 2hgo + 180 kj → 2hg + o2.
Mercuric oxide hgo decomposes to mercury on being heated which condenses on the cool parts of the test tube forming a silver mirror.
0.06 milligrams of mercury vapor per hour for every square centimeter of surface area.). Mercury(ii) oxide, a red solid, decomposes when heated to produce mercury and oxygen gas. This reaction results in the creation of mercuric oxide, an orange or red compound. Heating produces highly toxic mercury fumes and oxygen, which increases the fire hazard. Its vapour can produce harmful effects on the nervous, digestive and immune systems, lungs and kidenies, and may be fatal. Hgo decomposes on exposure to light or on heating above 500 °c.
 Source: fphoto.photoshelter.com
Source: fphoto.photoshelter.com
A reaction is also considered to be a decomposition reaction even when one or more of the products are still compounds. But please do not do this ! 0.06 milligrams of mercury vapor per hour for every square centimeter of surface area.). A chemical reaction happens when mercury is heated and reacts with oxygen. When heated to decomposition (932 f) it decomposes into mercury and oxygen.
 Source: fphoto.photoshelter.com
Source: fphoto.photoshelter.com
Action of heat on mercuric oxide: Mercury(ii) oxide, also called mercuric oxide or simply mercury oxide, is the inorganic compound with the formula hg o. Like most liquids they tend to evaporate if you heat them. It is mercury ii oxide or mercury i oxide. Mercury(ii) oxide is a red solid.
 Source: fphoto.photoshelter.com
Source: fphoto.photoshelter.com
Heating produces highly toxic mercury fumes and oxygen, which increases the fire hazard. A chemical reaction happens when mercury is heated and reacts with oxygen. A reaction is also considered to be a decomposition reaction even when one or more of the products are still compounds. Mercury(ii) oxide, also called mercuric oxide or simply mercury oxide, is the inorganic compound with the formula hg o. 0.06 milligrams of mercury vapor per hour for every square centimeter of surface area.).
 Source: alamy.com
Source: alamy.com
A reaction is also considered to be a decomposition reaction even when one or more of the products are still compounds. When it is heated to a temperature above 500c it easily decomposes into mercury and oxygen gas. Action of heat on mercuric oxide: The color change is the sign that the reaction is occurring. Mercuric oxide → heating mercury + oxygen.
 Source: kodabar.blogspot.com
Source: kodabar.blogspot.com
Mercuric oxide → heating mercury + oxygen. This reaction results in the creation of mercuric oxide, an orange or red compound. The color change is the sign that the reaction is occurring. Similarly, mercury reacts with oxidizing acids, such as nitric acid and concentrated sulfuric acid. Mercury(ii) oxide is a red solid.
 Source: fphoto.photoshelter.com
Source: fphoto.photoshelter.com
Mercury(ii) oxide is a red solid. What happens to mercury oxide is heated? The color change is the sign that the reaction is occurring. A chemical reaction happens when mercury is heated and reacts with oxygen. When we heat mercury oxide it decompose into metal and oxygen gas.
 Source: fphoto.photoshelter.com
Source: fphoto.photoshelter.com
The red color of the mercury oxide reactant becomes the silver color of mercury. Mercuric oxide → heating mercury + oxygen. When it is heated, it decomposes into mercury metal and oxygen gas. A chemical reaction happens when mercury is heated and reacts with oxygen. But please do not do this !
 Source: fphoto.photoshelter.com
Source: fphoto.photoshelter.com
Similarly, mercury reacts with oxidizing acids, such as nitric acid and concentrated sulfuric acid. Mercury(ii) oxide, also called mercuric oxide or simply mercury oxide, is the inorganic compound with the formula hg o. Heating produces highly toxic mercury fumes and oxygen, which increases the fire hazard. What happens when mercuric oxide is heated? Mercury(ii) oxide, a red solid, decomposes when heated to produce mercury and oxygen gas.
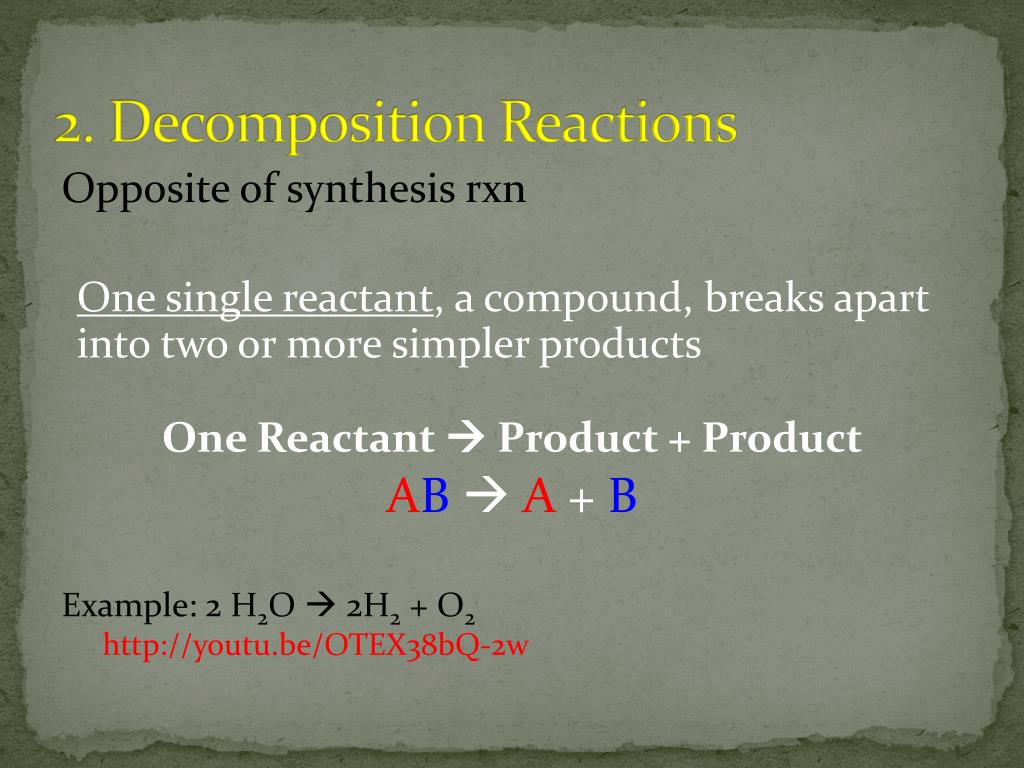 Source: slideserve.com
Source: slideserve.com
The red color of the mercury oxide reactant becomes the silver color of mercury. Mercuric oxide → heating mercury + oxygen. When heated mercury reacts oxygen in the air to form mercury oxide, which undergoes decomposition upot heating to higher temperature. What happens when you mix mercury and oxygen? It is mercury ii oxide or mercury i oxide.
 Source: fphoto.photoshelter.com
Source: fphoto.photoshelter.com
Heating produces highly toxic mercury fumes and oxygen, which increases the fire hazard. 0.06 milligrams of mercury vapor per hour for every square centimeter of surface area.). Heating produces highly toxic mercury fumes and oxygen, which increases the fire hazard. But please do not do this ! Similarly, mercury reacts with oxidizing acids, such as nitric acid and concentrated sulfuric acid.
 Source: slideshare.net
Source: slideshare.net
When heated to decomposition (932 f) it decomposes into mercury and oxygen. Heating produces highly toxic mercury fumes and oxygen, which increases the fire hazard. Similarly, mercury reacts with oxidizing acids, such as nitric acid and concentrated sulfuric acid. When we heat mercury oxide it decompose into metal and oxygen gas. The color change is the sign that the reaction is occurring.
 Source: fphoto.photoshelter.com
Source: fphoto.photoshelter.com
The color change is the sign that the reaction is occurring. A reaction is also considered to be a decomposition reaction even when one or more of the. Mercury(ii) oxide, also called mercuric oxide or simply mercury oxide, is the inorganic compound with the formula hg o. Similarly, mercury reacts with oxidizing acids, such as nitric acid and concentrated sulfuric acid. When it is heated, it decomposes into mercury metal and oxygen gas.
 Source: fphoto.photoshelter.com
Source: fphoto.photoshelter.com
This reaction results in the creation of mercuric oxide, an orange or red compound. When heated to decomposition (932 f) it decomposes into mercury and oxygen. What happens when mercuric oxide is heated? Like most liquids they tend to evaporate if you heat them. A reaction is also considered to be a decomposition reaction even when one or more of the.
 Source: shaunmwilliams.com
Source: shaunmwilliams.com
When heated mercury reacts oxygen in the air to form mercury oxide, which undergoes decomposition upot heating to higher temperature. Mercuric oxide → heating mercury + oxygen. What happens when mercuric oxide is heated? It may be collected over mercury. When it is heated, it decomposes into mercury metal and oxygen gas.
 Source: fphoto.photoshelter.com
Source: fphoto.photoshelter.com
Action of heat on mercuric oxide: A chemical reaction happens when mercury is heated and reacts with oxygen. This reaction results in the creation of mercuric oxide, an orange or red compound. Heating produces highly toxic mercury fumes and oxygen, which increases the fire hazard. What happens when mercuric oxide is heated?
 Source: fphoto.photoshelter.com
Source: fphoto.photoshelter.com
This reaction results in the creation of mercuric oxide, an orange or red compound. When heated mercury reacts oxygen in the air to form mercury oxide, which undergoes decomposition upot heating to higher temperature. When it is heated in a test tube, it decomposes to give mercury and oxygen. Mercury(ii) oxide, a red solid, decomposes when heated to produce mercury and oxygen gas. It may be collected over mercury.
 Source: fphoto.photoshelter.com
Source: fphoto.photoshelter.com
Similarly, mercury reacts with oxidizing acids, such as nitric acid and concentrated sulfuric acid. This reaction results in the creation of mercuric oxide, an orange or red compound. When heated mercury reacts oxygen in the air to form mercury oxide, which undergoes decomposition upot heating to higher temperature. What happens to mercury (ii) oxide when heated? 2 hgo 2 hg + o a 2.
 Source: fphoto.photoshelter.com
Source: fphoto.photoshelter.com
Heating produces highly toxic mercury fumes and oxygen, which increases the fire hazard. It is mercury ii oxide or mercury i oxide. It has a red or orange color. Mercury(ii) oxide is a red solid. What happens when you mix mercury and oxygen?
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title what happens when mercury oxide is heated by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.