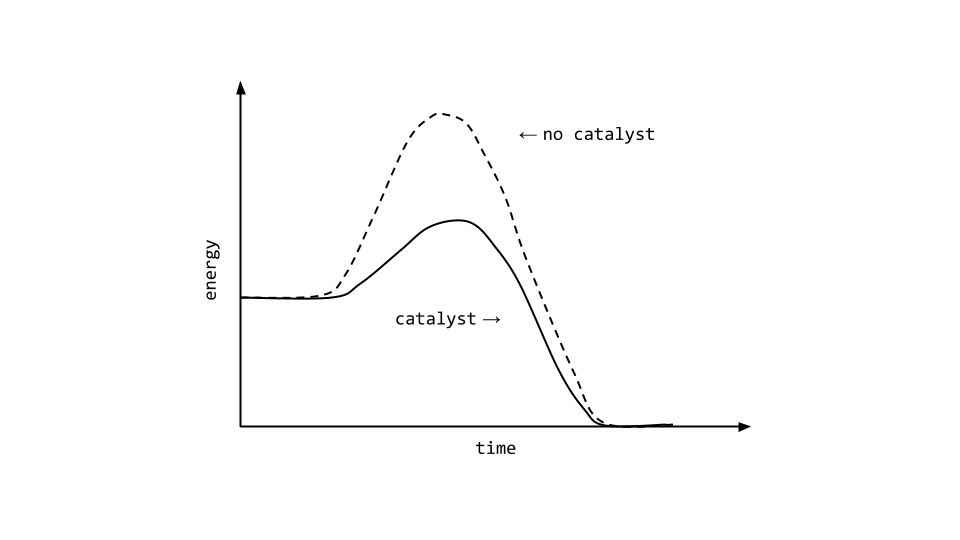
What is ground state electron configuration
What Is Ground State Electron Configuration. So, it needs to lose one electron to attain stability and get an electronic configuration like noble gas helium. Shorthand electron configuration full electron configuration electron shell arrangement; This is the lowest energy or ground state electron configuration. Many scientists worked to give exact electron configuration in of specific atoms.
 Electrons in Their GroundState Electron Configurations From easychem.com.au
Electrons in Their GroundState Electron Configurations From easychem.com.au
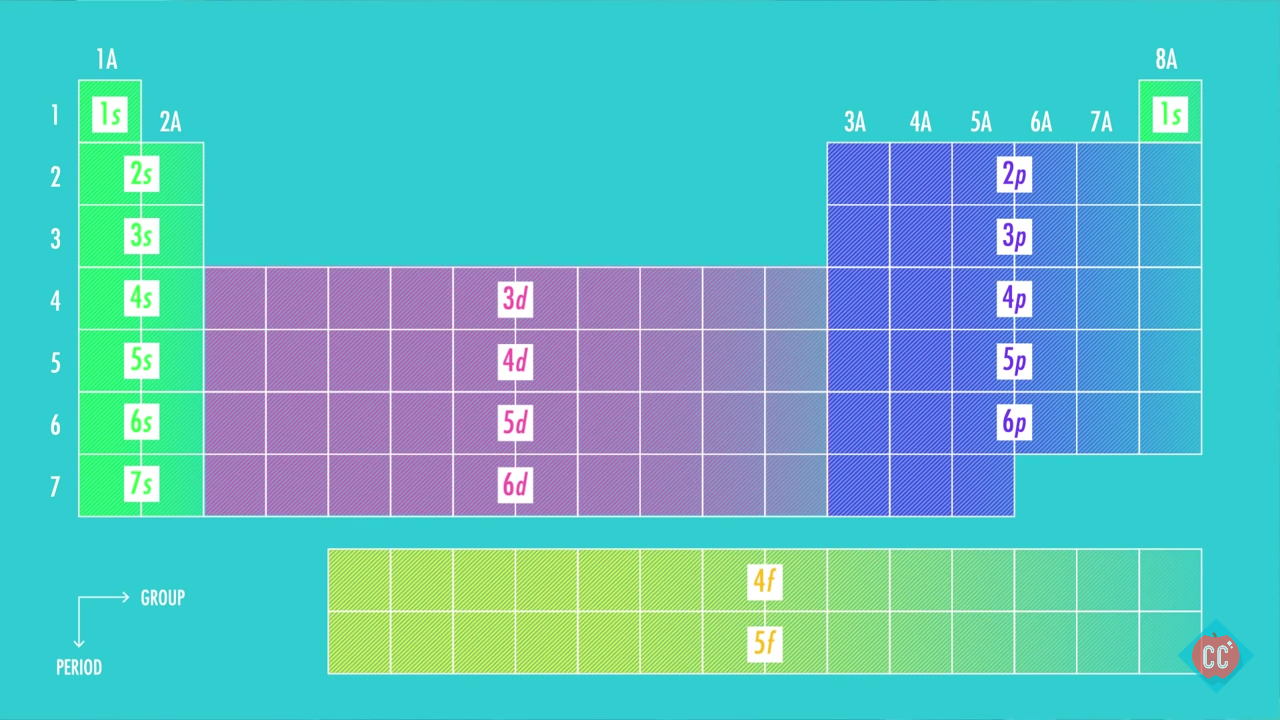
The aufbau principle states that, in a ground state electron configuration, the lowest energy orbitals are filled with electrons first. Electron configurations can be determined using a periodic table. A shorthand way to write the electron configuration, called noble gas notation, is [ne. The ground state electronic configuration of scandium is [ar] 3d1 4s2. So, it needs to lose one electron to attain stability and get an electronic configuration like noble gas helium. It has immense importance in knowing valency of an atom or molecules in chemical reactions.
Ground state electron configuration of sodium (na):
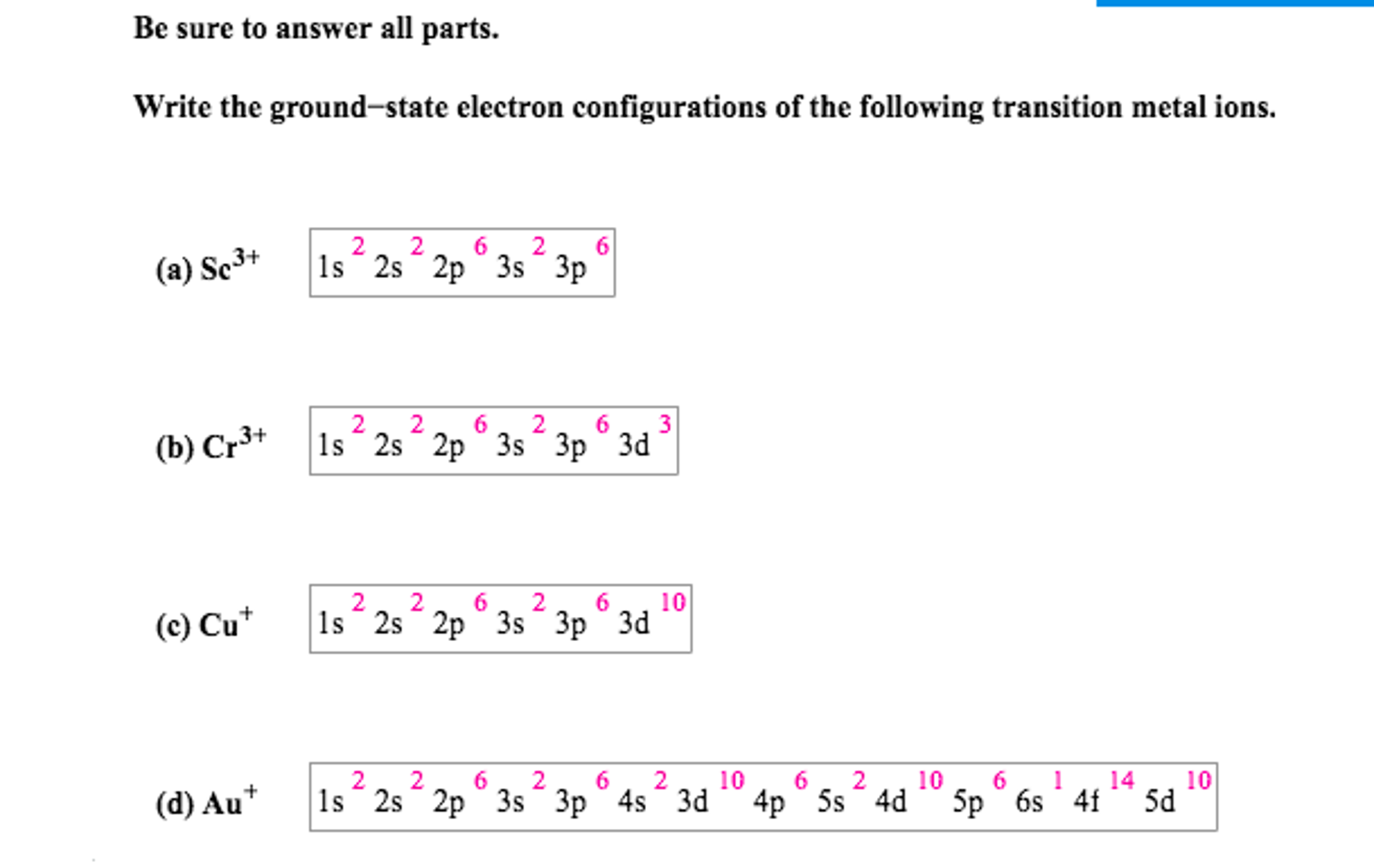
Thus, its valency is 1. Atomic number, atomic weight and charge of iron ion. Ground state electron configuration is a systematic distribution of electrons in atomic orbits of an atom or element. Electron configuration of hydrogen (h) 1s 1: The electronic configuration of zinc in the ground state is 1 s 2 2 s 2 2 p 6 3 s. The atomic number of aluminum is 13.
 Source: ochempal.org
Source: ochempal.org
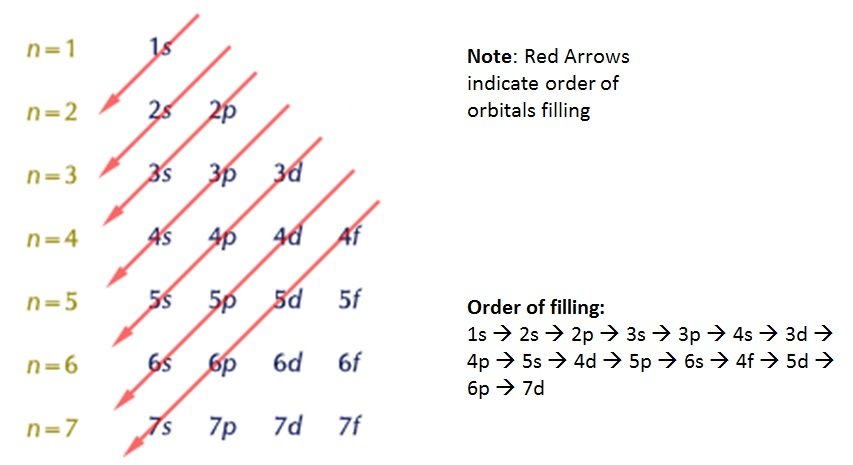
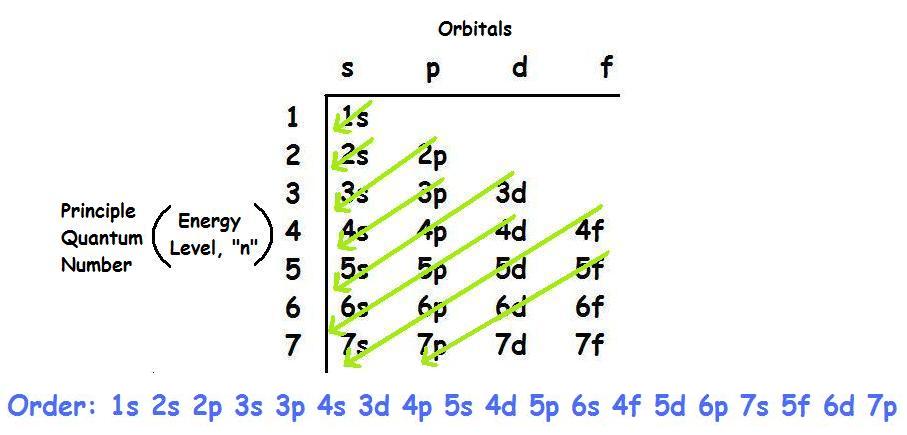
This is the lowest energy or ground state electron configuration. The numbers with s come first following p and d and f. The ground state electronic configuration of scandium is [ar] 3d1 4s2. Electron configuration of hydrogen (h) 1s 1: What is ground state electron configuration of atoms.
 Source: socratic.org
Source: socratic.org
A shorthand way to write the electron configuration, called noble gas notation, is [ne. We tried to answer the question “ what is the. Atomic number, atomic weight and charge of iron ion. The ground state electron configuration for aluminum is 1s 2 2s 2 2p 6 3s 2 3p 1. This implies that the neutral ge atom�s electron configuration must account for 32 electrons.
 Source: study.com
Source: study.com
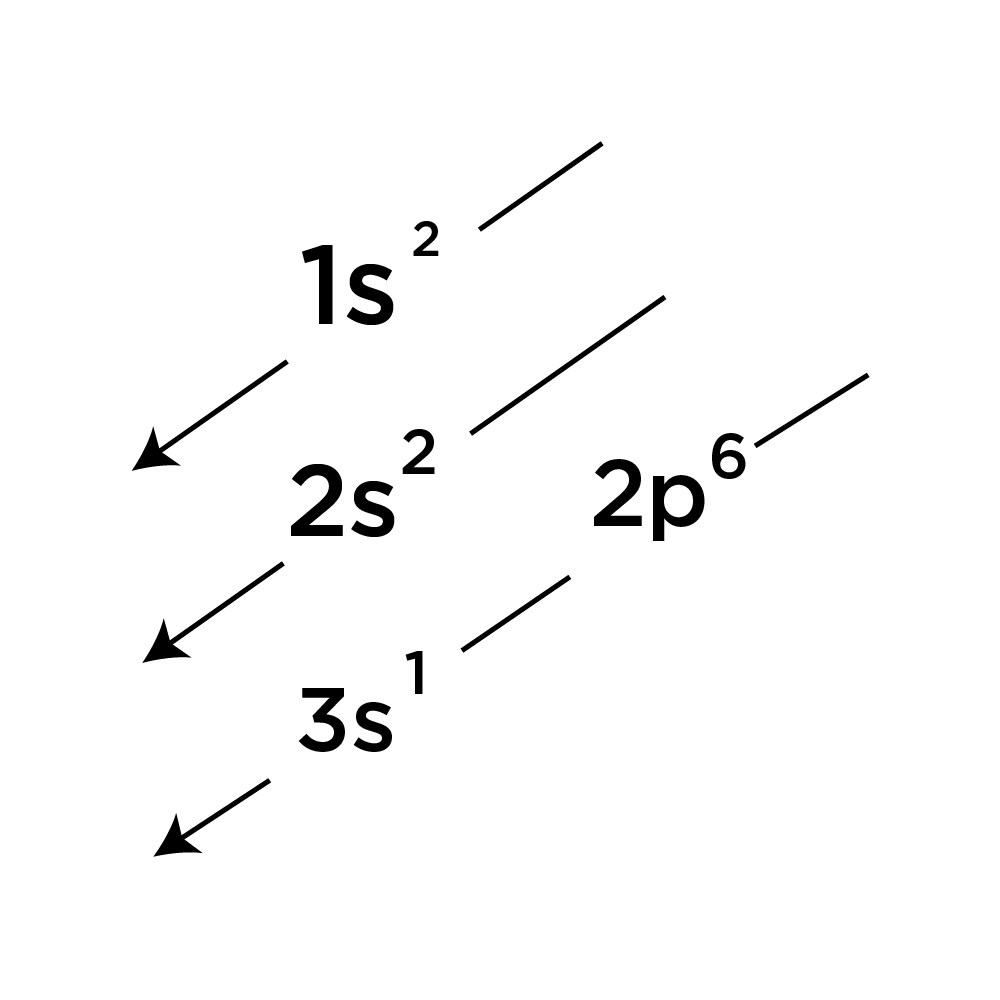
We found that an electron in an atom in the periodic table has four quantum numbers— n, l, m (l) , and m (s)—associated with it. Electron configuration of lithium (li) [he] 2s 1: A shorthand way to write the electron configuration, called noble gas notation, is [ne. By following the diagonal arrow method you can easily write down the ground state electron configuration of sodium (na) 1s 2 2s 2 2p 6 3s 1. We tried to answer the question “ what is the.
 Source: youtube.com
Source: youtube.com
The electronic configuration of zinc in the ground state is 1 s 2 2 s 2 2 p 6 3 s. Atomic number, atomic weight and charge of iron ion. What is ground state electron configuration of atoms. In this lecture, we proceed with the discussion of quantum figures, their use in electron setup, and the connection of ground state electron configuration to the periodic residential properties of the components. Electron configuration of lithium (li) [he] 2s 1:
 Source: easychem.com.au
Source: easychem.com.au
In this lecture, we proceed with the discussion of quantum figures, their use in electron setup, and the connection of ground state electron configuration to the periodic residential properties of the components. Electron configuration of helium (he) 1s 2: Electron configuration of hydrogen (h) 1s 1: The ground state electronic configuration of scandium is [ar] 3d1 4s2. We tried to answer the question “ what is the.
 Source: orangatame.com
Source: orangatame.com
Electron configuration of beryllium (be) [he] 2s 2: The web content that complies with is the substance of general chemistry lecture 26. An alternative way of writing the electron configuration for ge is by using the noble gas shorthand notation. The ground state electronic configuration of scandium is [ar] 3d1 4s2. What is ground state electron configuration of atoms.
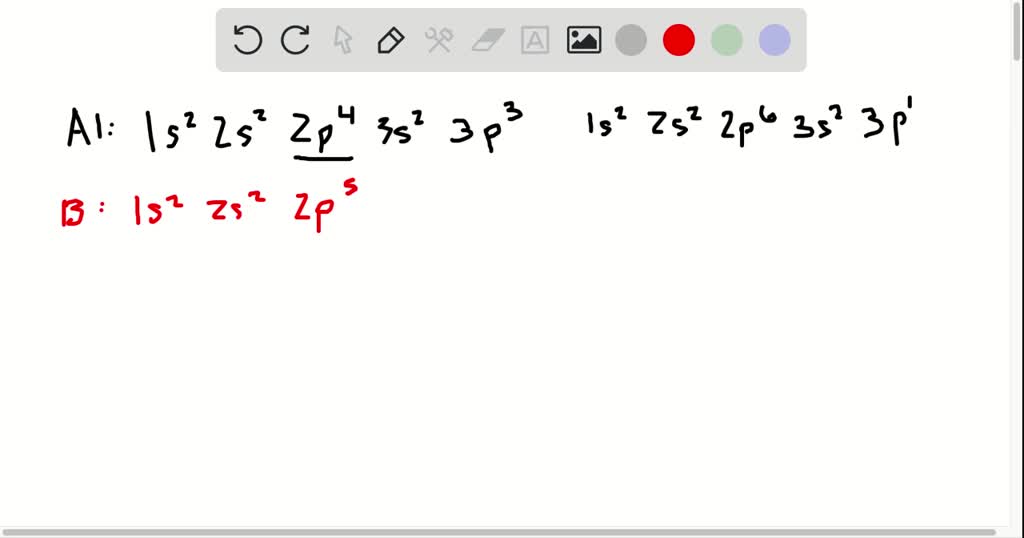 Source: numerade.com
Source: numerade.com
An alternative way of writing the electron configuration for ge is by using the noble gas shorthand notation. “n” is the principal quantum number, “l” is the angular quantum number, “m (l)” is the magnetic quantum number, and “m (s)” is the spin quantum. Electronic configuration of zinc zn: Electron configuration of helium (he) 1s 2: The electronic configuration of an element is written with the help of afbau�s principle, which states that the lower energy states are first filled completely, then the.
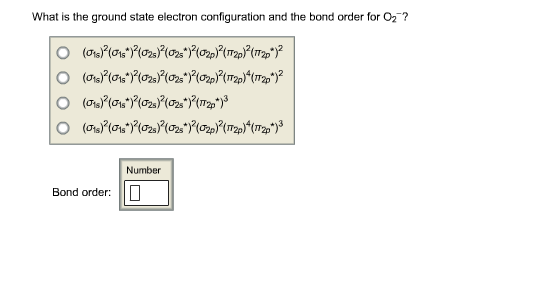 Source: chegg.com
Source: chegg.com
The web content that complies with is the substance of general chemistry lecture 26. The ground state electronic configuration of scandium is [ar] 3d1 4s2. >> back to key information about the. This is the lowest energy or ground state electron configuration. The electronic configuration of zinc in the ground state is 1 s 2 2 s 2 2 p 6 3 s.
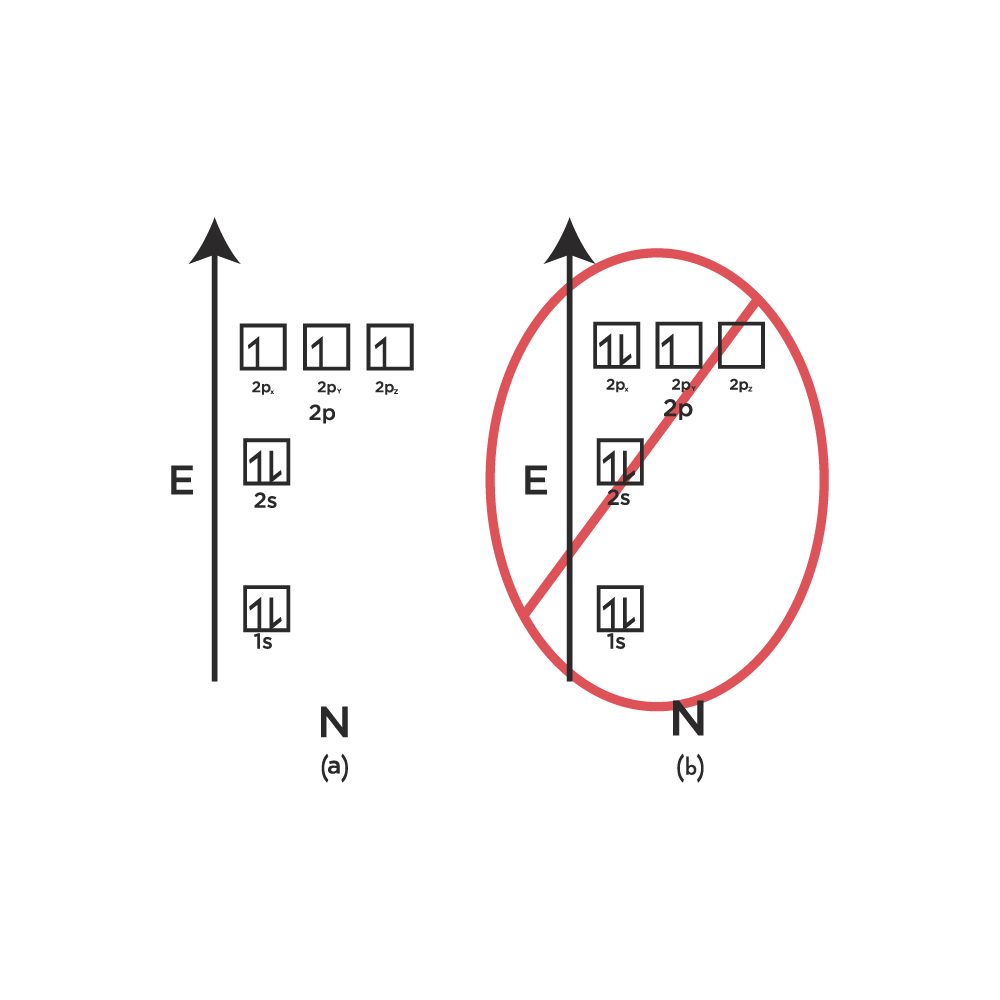 Source: orangatame.com
Source: orangatame.com
The electron configuration of molybdenum in its ground state is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d5 5s1. Electron configuration of hydrogen (h) 1s 1: Electron configuration of beryllium (be) [he] 2s 2: The ground state electronic configuration of scandium is [ar] 3d1 4s2. In this lecture, we proceed with the discussion of quantum figures, their use in electron setup, and the connection of ground state electron configuration to the periodic residential properties of the components.
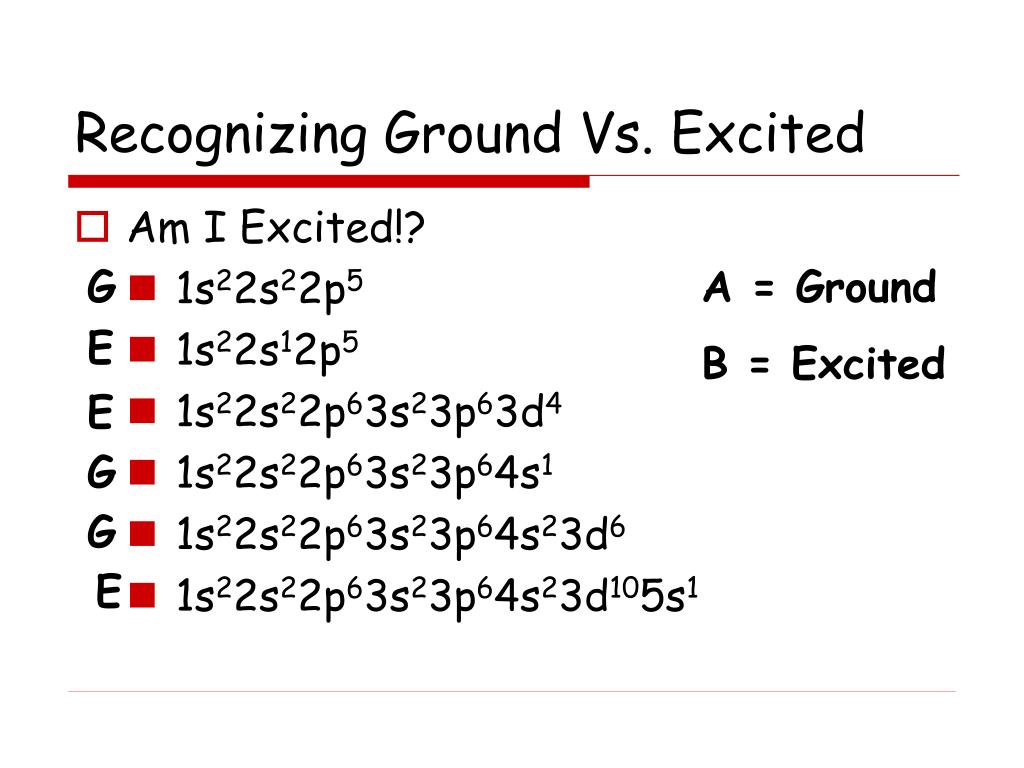 Source: xepisty.blogspot.com
Source: xepisty.blogspot.com
We found that an electron in an atom in the periodic table has four quantum numbers— n, l, m (l) , and m (s)—associated with it. The atomic number of aluminum is 13. The ground state electronic configuration of scandium is [ar] 3d1 4s2. Electronic configuration of zinc zn: When writing down the configuration remember the order of s, p, d, f.
 Source: chadsprep.com
Source: chadsprep.com
Ground state electron configuration is a systematic distribution of electrons in atomic orbits of an atom or element. Atomic number, atomic weight and charge of iron ion. An alternative way of writing the electron configuration for ge is by using the noble gas shorthand notation. In this lecture, we proceed with the discussion of quantum figures, their use in electron setup, and the connection of ground state electron configuration to the periodic residential properties of the components. The atomic number of aluminum is 13.
 Source: electronschematic.blogspot.com
Source: electronschematic.blogspot.com
Electron configurations can be determined using a periodic table. So, it needs to lose one electron to attain stability and get an electronic configuration like noble gas helium. A neutral atom of aluminum has 13 protons and 13 electrons. Oxygen ion’s bond order can be calculated by this formula: The ground state electron configuration is the arrangement of electrons around the nucleus of an atom with lower energy levels.
 Source: chegg.com
Source: chegg.com
Germanium ( ge) is located in the fourth row, group 14 of the periodic table, and has an atomic number of 32. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6. In a neutral atom, the number of electrons is the same as the number of protons. >> back to key information about the. A neutral atom of aluminum has 13 protons and 13 electrons.
 Source: orangatame.com
Source: orangatame.com
1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6. Shorthand electron configuration full electron configuration electron shell arrangement; It has immense importance in knowing valency of an atom or molecules in chemical reactions. Oxygen ion’s bond order can be calculated by this formula: This implies that the neutral ge atom�s electron configuration must account for 32 electrons.
 Source: socratic.org
Source: socratic.org
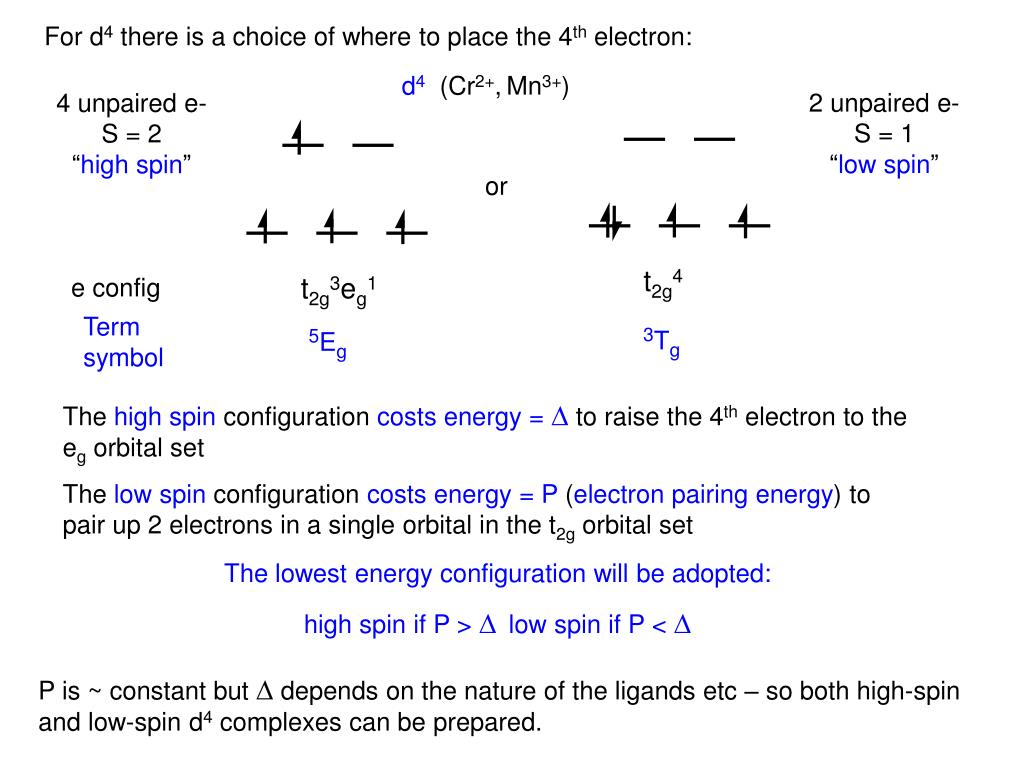
The electronic configuration of an element is written with the help of afbau�s principle, which states that the lower energy states are first filled completely, then the. So, it needs to lose one electron to attain stability and get an electronic configuration like noble gas helium. The same goes for chromium (right above molydenum), and the group containing gold, silver and copper. We found that an electron in an atom in the periodic table has four quantum numbers— n, l, m (l) , and m (s)—associated with it. The ground state electron configuration is the arrangement of electrons around the nucleus of an atom with lower energy levels.
 Source: socratic.org
Source: socratic.org
An alternative way of writing the electron configuration for ge is by using the noble gas shorthand notation. This is the lowest energy or ground state electron configuration. Oxygen ion’s bond order can be calculated by this formula: Electronic configuration of zinc zn: >> back to key information about the.
 Source: orangatame.com
Source: orangatame.com
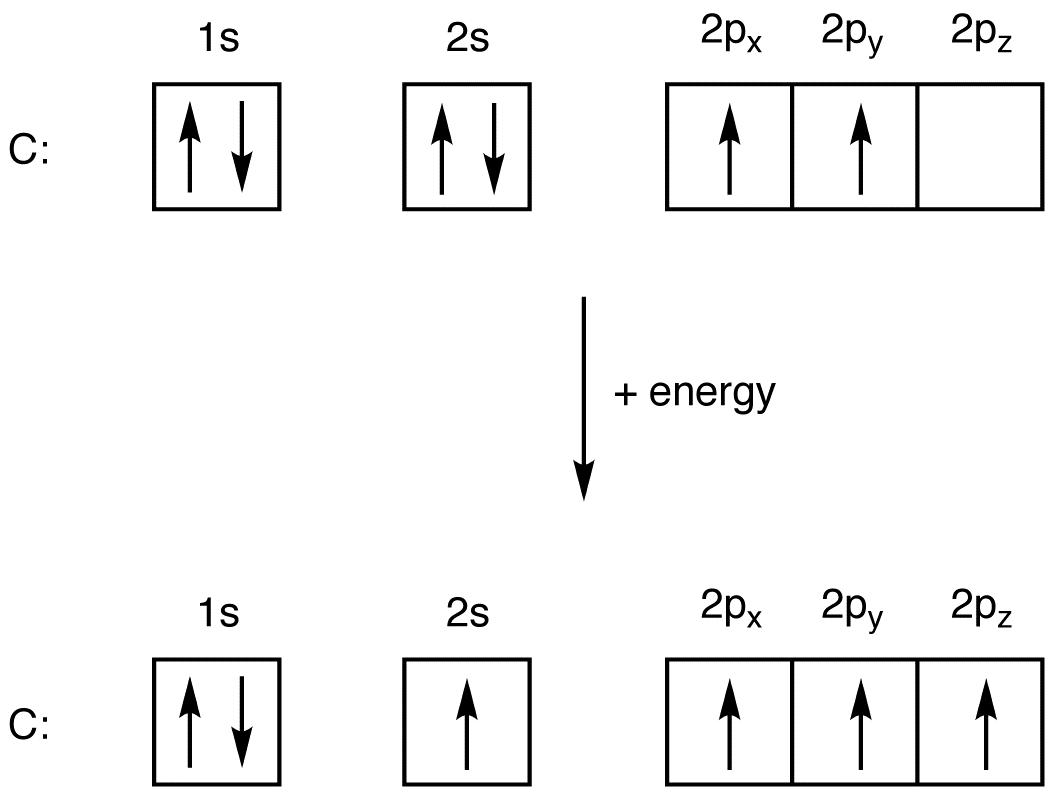
Electron configuration of beryllium (be) [he] 2s 2: Electronic configuration of zinc zn: Electron configuration of lithium (li) [he] 2s 1: In a neutral atom, the number of electrons is the same as the number of protons. The two homos have the same energy and are half filled with one electron in each;
 Source: iperiodictable.com
Source: iperiodictable.com
The electron configuration of molybdenum in its ground state is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d5 5s1. The numbers with s come first following p and d and f. What is ground state electron configuration of atoms. The ground state configuration is the lowest energy, most stable arrangement. Electron configuration of second period elements showing orbital notation and electron configuration notation:
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title what is ground state electron configuration by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.