What is the relationship between temperature and kinetic energy
What Is The Relationship Between Temperature And Kinetic Energy. As a subtance absorbs heat the particles move faster so the average kinetic energy and therefore the temperature increases. Temperatures also measure how kinetic energy is not how hot or cold it is. Kinetic energy is the energy that an object has because of its motion. Temperature is directly proportional to the kinetic energy of the atoms that a body is made of.
 Chemistry Graphs Distribution of Energies From algebralab.org
Chemistry Graphs Distribution of Energies From algebralab.org
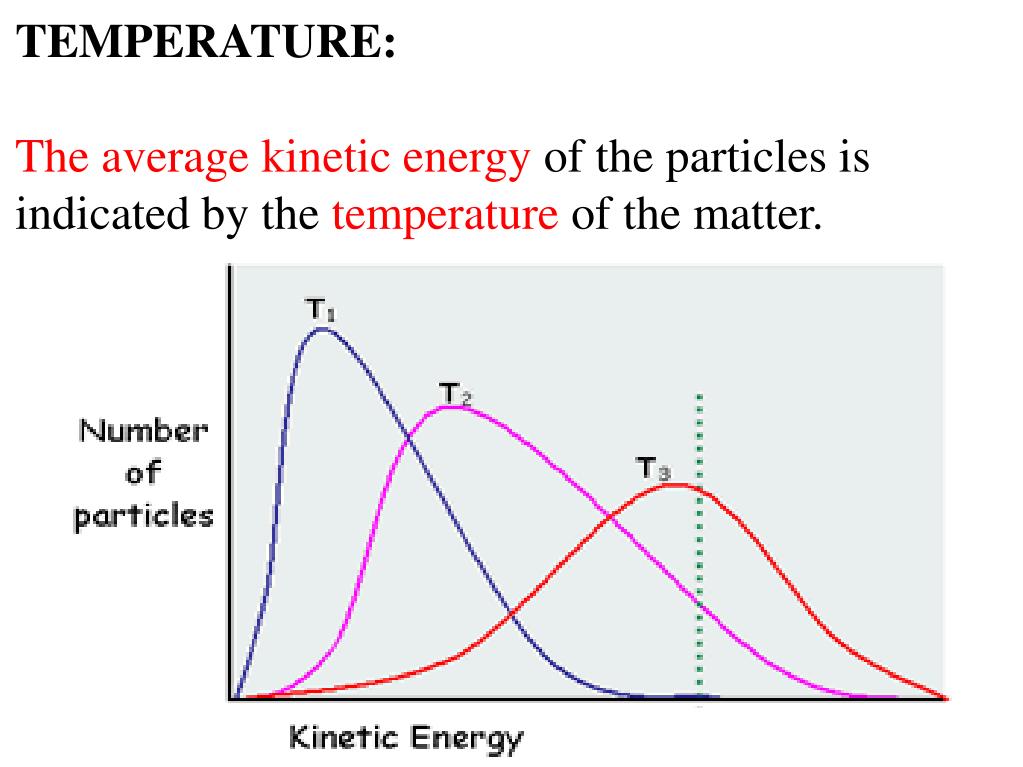
As molecules move more quickly, the temperature increases. The average kinetic energy of molecules/particles of the hotter body is more than the colder body. This relation is valid concerning the velocities relative to the center of mass of the body. Because kinetic energy is the energy of a substance contained because of its molecules existing in activity, as a substance consumes heat its molecules shift quickly, thereby improving the substance�s. Kinetic theory gases postulates equation derivation. Which statement best describes the relationship between temperature and kinetic energy of particles?
In physics, the kinetic energy of an object is the energy that it possesses due to its motion.
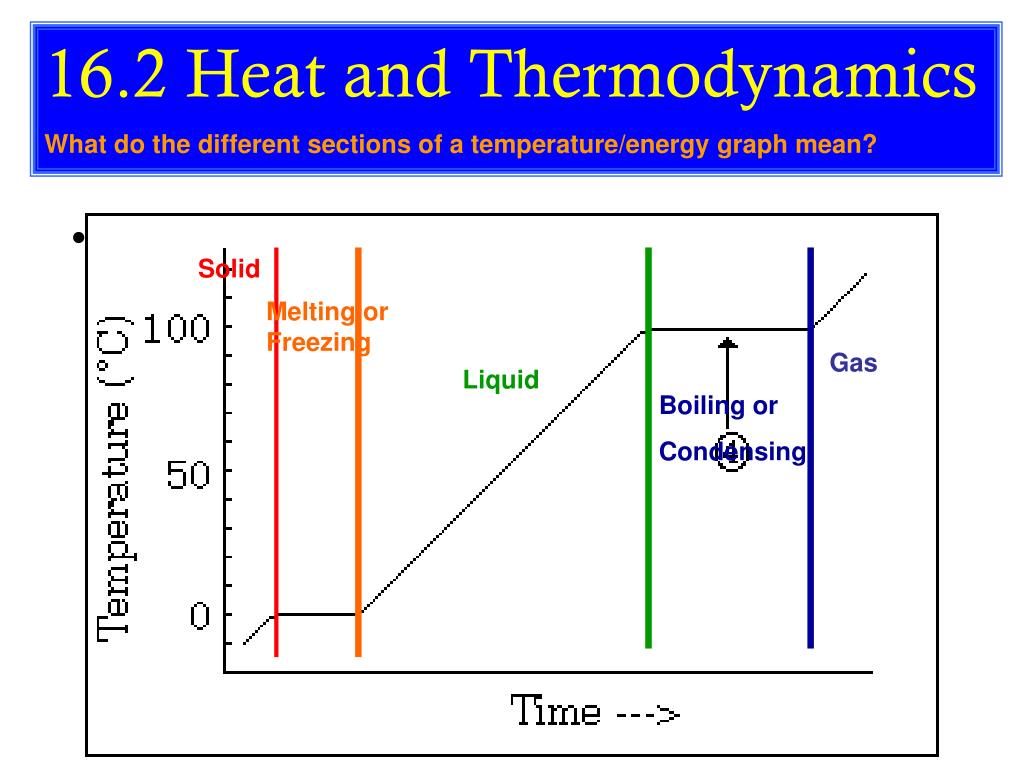
The measurement of temperature is the resultant impact of the exchange of kinetic energy between the system and the molecules or atoms within measuring instrument (ie. The association between temperature and kinetic energy is defined as follows:. Temperature is the average kinetic energy of a substance, and a measure of how hot or cold something is. Temperature is directly proportional to the kinetic energy of the atoms that a body is made of. Temperature is proportional to heat and is the measure of the average kinetic energy of the molecules in a system. As molecules slow down, the temperature starts to increase.
 Source: frazerphysics.blogspot.com
Source: frazerphysics.blogspot.com
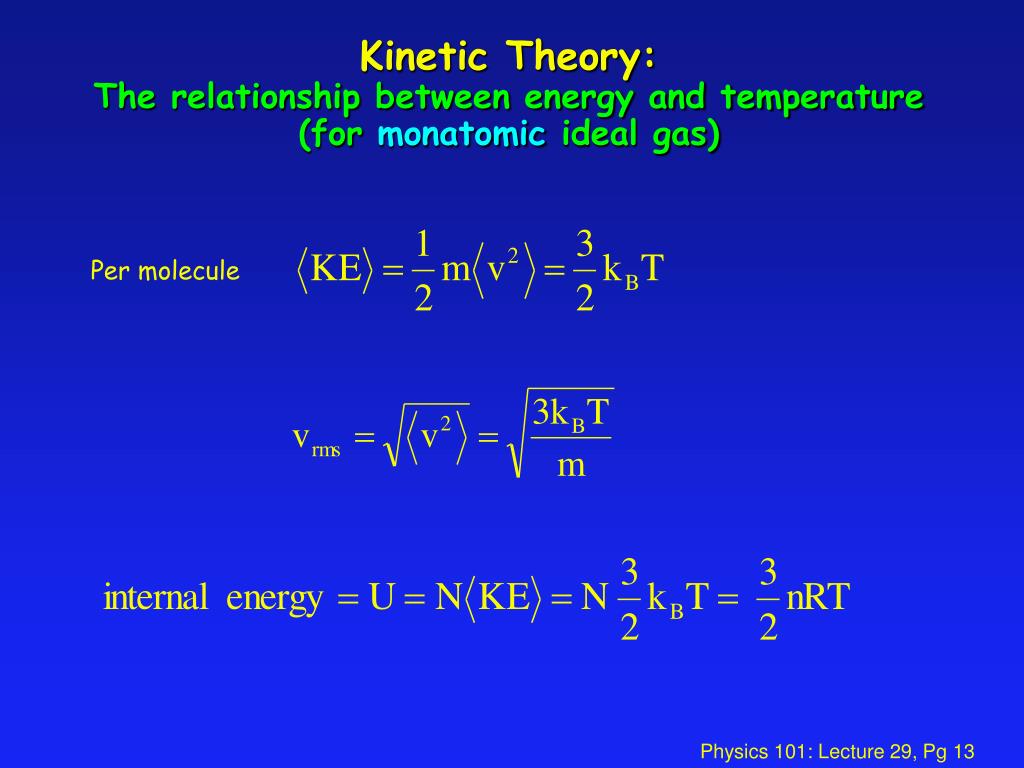
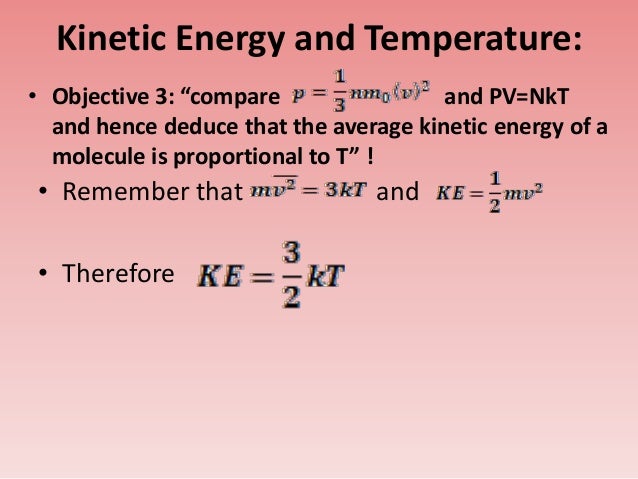
In other words, concerted movements like translation and rotation do. When all molecular motion stops, the temperature starts to decrease d. See answer (1) best answer. For ideal gas(pv=nkbt), i can derive the relationship between temperature and kinetic energy. Temperature is a measurement of the average kinetic energy of the molecules in an object or a system.
 Source: chegg.com
Source: chegg.com
The association between temperature and kinetic energy is defined as follows:. What is the relationship between temperature and kinetic energy? Kinetic energy is a function of temperature. When all molecular motion stops, the temperature stops changing. The measurement of temperature is the resultant impact of the exchange of kinetic energy between the system and the molecules or atoms within measuring instrument (ie.
 Source: algebralab.org
Source: algebralab.org
Temperatures also measure how kinetic energy is not how hot or cold it is. For ideal gas(pv=nkbt), i can derive the relationship between temperature and kinetic energy. The measurement of temperature is the resultant impact of the exchange of kinetic energy between the system and the molecules or. Temperatures also measure how kinetic energy is not how hot or cold it is. The key difference between kinetic energy and temperature is that kinetic energy refers to the property of a moving object, specifically the work needed to accelerate a body from its resting state, whereas temperature is the thermal energy present in all matter.
 Source: slideserve.com
Source: slideserve.com
Derive the relation between kinetic temperature thermal energy of a gas molecule falling object what is article average molecular theory relationship static pressure and thermodynamic wikipedia. The association between temperature and kinetic energy is defined as follows:. The average kinetic energy of molecules/particles of the hotter body is more than the colder body. Kinetic energy and temperature are related terms because the kinetic energy of a system can. Temperature is the average kinetic energy of a substance, and a measure of how hot or cold something is.
 Source: slideserve.com
Source: slideserve.com
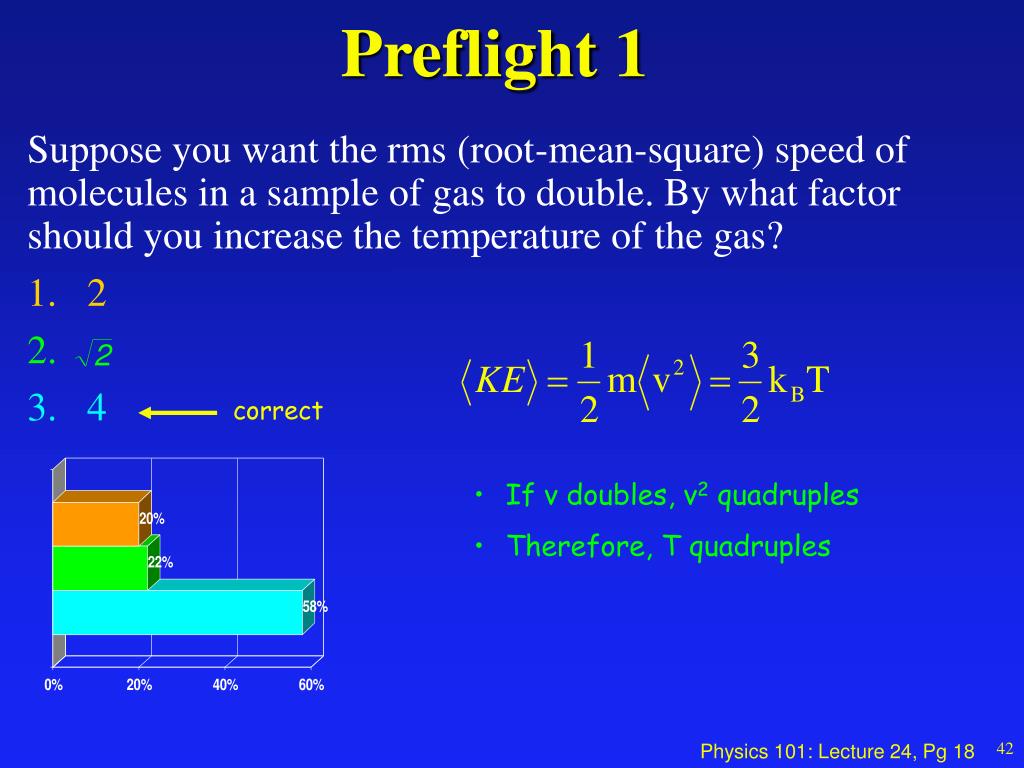
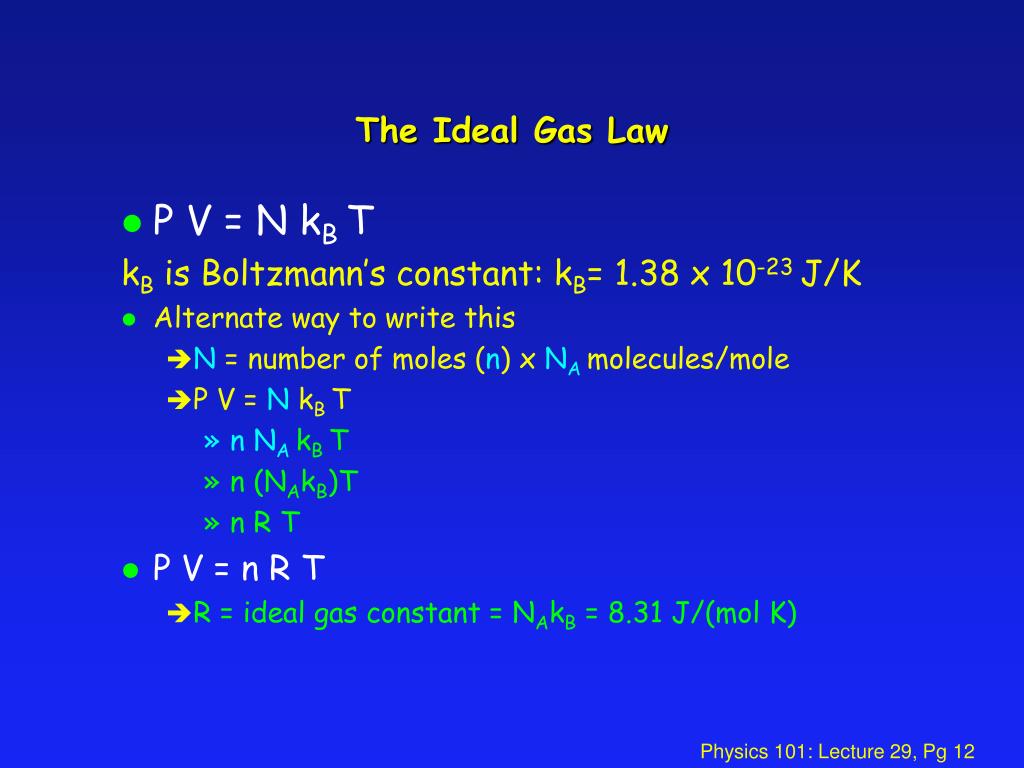
In physics, the kinetic energy of an object is the energy that it possesses due to its motion. Kinetic energy and temperature are related terms because the kinetic energy of a system can. Ch 14 the ideal gas law and kinetic. Kinetic energy is the energy that an object has because of its motion. When all molecular motion stops, the temperature stops changing.
 Source: slideshare.net
Source: slideshare.net
Temperatures also measure how kinetic energy is not how hot or cold it is. Temperature is proportional to heat and is the measure of the average kinetic energy of the molecules in a system. Temperature is the average kinetic energy of a substance, and a measure of how hot or cold something is. As molecules slow down, the temperature starts to increase. Kinetic energy is a function of temperature.
 Source: slideserve.com
Source: slideserve.com
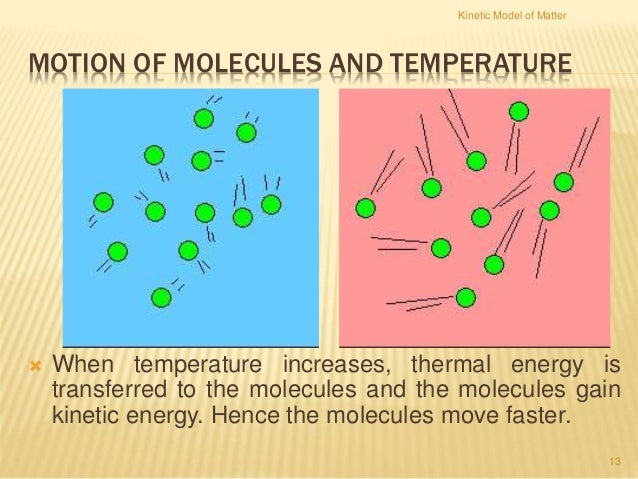
Kinetic energy is the energy that an object has because of its motion. When all molecular motion stops, the temperature stops changing. In md simulations, we can calculate the temperature using the average of kinetic energy of the system. As molecules move more quickly, the temperature increases. Kinetic energy is the energy that an object has because of its motion.
 Source: slideserve.com
Source: slideserve.com
As molecules move more quickly, the temperature increases. Because kinetic energy is the energy of a substance contained because of its molecules existing in activity, as a substance consumes heat its molecules shift quickly, thereby improving the substance�s. For ideal gas(pv=nkbt), i can derive the relationship between temperature and kinetic energy. When all molecular motion stops, the temperature starts to decrease d. The molecules in a substance have a range of kinetic energies because they don’t all move at the same speed.
Source: go.isptutor.org
The key difference between kinetic energy and temperature is that kinetic energy refers to the property of a moving object, specifically the work needed to accelerate a body from its resting state, whereas temperature is the thermal energy present in all matter. As molecules move more quickly, the temperature increases. When all molecular motion stops, the temperature starts to decrease d. As the kinetic energy of a substance. Because kinetic energy is the energy of a substance contained because of its molecules existing in activity, as a substance consumes heat its molecules shift quickly, thereby improving the substance�s.
 Source: chegg.com
Source: chegg.com
The molecules in a substance have a range of kinetic energies because they don’t all move at the same speed. Temperature is the average kinetic energy of a substance, and a measure of how hot or cold something is. This relation is valid concerning the velocities relative to the center of mass of the body. The measurement of temperature is the resultant impact of the exchange of kinetic energy between the system and the molecules or atoms within measuring instrument (ie. What is the relationship between temperature and kinetic energy?
 Source: slideserve.com
Source: slideserve.com
What kind of relationship exists between kinetic energy and temperature? Kinetic energy is a function of temperature. Kinetic molecular theory of gases khan academy. As molecules slow down, the temperature starts to increase. The measurement of temperature is the resultant impact of the exchange of kinetic energy between the system and the molecules or.
 Source: tessshebaylo.com
Source: tessshebaylo.com
Temperature is the average kinetic energy of a substance, and a measure of how hot or cold something is. What kind of relationship exists between kinetic energy and temperature? What is the relationship between temperature and kinetic energy? Kinetic energy is the energy that an object has because of its motion. As the kinetic energy of a substance.
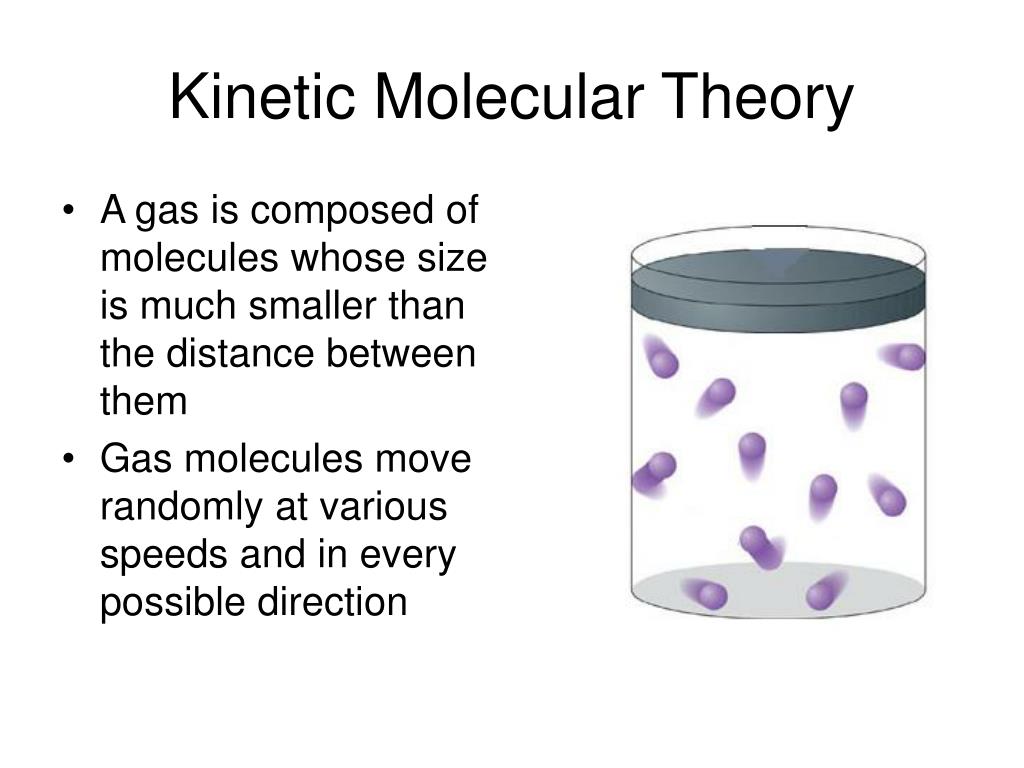 Source: slideserve.com
Source: slideserve.com
The association between temperature and kinetic energy is defined as follows:. The key difference between kinetic energy and temperature is that kinetic energy refers to the property of a moving object, specifically the work needed to accelerate a body from its resting state, whereas temperature is the thermal energy present in all matter. What kind of relationship exists between kinetic energy and temperature? This relation is valid concerning the velocities relative to the center of mass of the body. Temperature is a measurement of the average kinetic energy of the molecules in an object or a system.
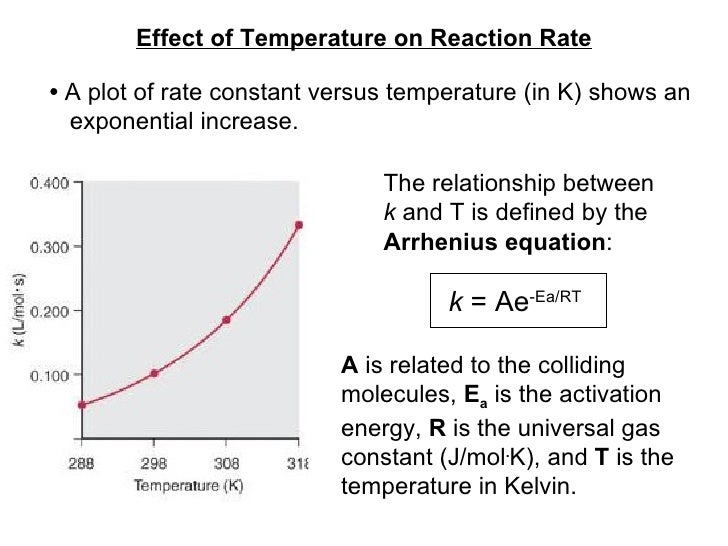 Source: slideshare.net
Source: slideshare.net
In physics, the kinetic energy of an object is the energy that it possesses due to its motion. In physics, the kinetic energy of an object is the energy that it possesses due to its motion. As molecules slow down, the temperature starts to increase. When all molecular motion stops, the temperature stops changing. What is the relationship between heat and kinetic energy?
 Source: reddit.com
Source: reddit.com
For ideal gas(pv=nkbt), i can derive the relationship between temperature and kinetic energy. As molecules slow down, the temperature starts to increase. As a subtance absorbs heat the particles move faster so the average kinetic energy and therefore the temperature increases. In physics, the kinetic energy of an object is the energy that it possesses due to its motion. As heat is introduced into a system, the molecules absorb the thermal energy and increase their kinetic energy.
 Source: youtube.com
Source: youtube.com
As molecules slow down, the temperature starts to increase. It is defined as the work needed to accelerate a body of a given mass from rest to its stated velocity.having gained this energy during its acceleration, the body maintains this kinetic energy unless its speed changes.the same amount of work is done by the body when decelerating. What is the relationship between temperature and kinetic energy? As a substance gains kinetic energy, its particles begin to move faster. Temperature is the average kinetic energy of a substance, and a measure of how hot or cold something is.
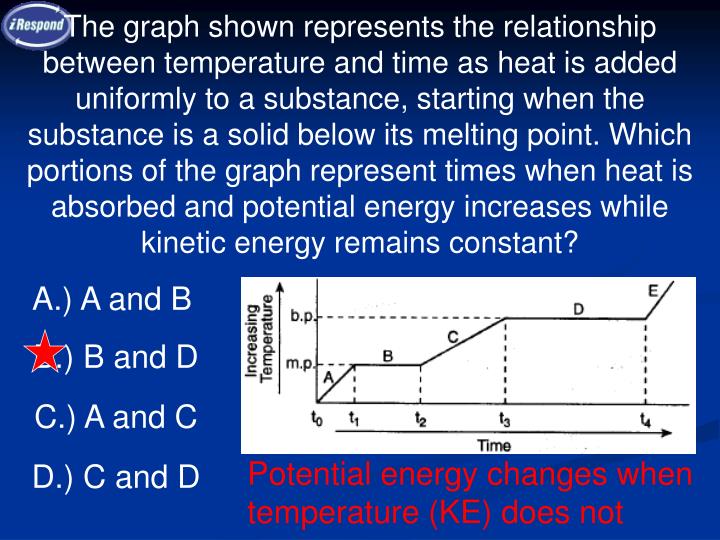 Source: slideserve.com
Source: slideserve.com
Kinetic molecular theory of gases khan academy. The molecules in a substance have a range of kinetic energies because they don’t all move at the same speed. What kind of relationship exists between kinetic energy and temperature? Temperatures also measure how kinetic energy is not how hot or cold it is. Temperature is directly proportional to the kinetic energy of the atoms that a body is made of.
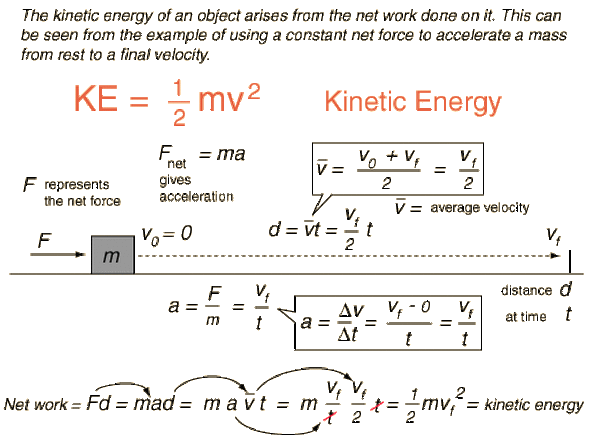 Source: superawsomeformsofenergy.weebly.com
Source: superawsomeformsofenergy.weebly.com
In physics, the kinetic energy of an object is the energy that it possesses due to its motion. Temperature is a measure of the average kinetic energy of the particles in an object. For ideal gas(pv=nkbt), i can derive the relationship between temperature and kinetic energy. Ch 14 the ideal gas law and kinetic. What is the relationship between heat and kinetic energy?
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title what is the relationship between temperature and kinetic energy by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.