What will lipids dissolve in
What Will Lipids Dissolve In. Lipids are a heterogeneous class of naturally occurring organic substances grouped together not by the presence of a distinguishing functional group or structural feature, but rather on the basis of common solubility properties. The functions of lipids include. Hence they are soluble in organic solvents such as ethanol (alcohol), but insoluble in water. It is moreover a human�s current understanding that it.
In the case of shield and our technologies, the detergent sodium dodecyl sulfate (sds) is used to dissolve the lipids in the cell membranes to improve tissue transparency. The hydrophobic interaction of the carbon in the short chain with water is not great and is overcome by the hydrogen bonding. Lipid solubility is an important and desired feature of most administered drugs that allows for the passive diffusion across cellular membranes. Ethanol extracts the lipid from the crushed solid sample. How to dissolve lipids in water? We recommend ensuring the precipitate has completely redissolved before using the solution (if possible).
What kinds of solvents can lipids dissolve in and why?
The hydrophobic interaction of the carbon in the short chain with water is not great and is overcome by the hydrogen bonding. Solubility of lipids in organic solvents is dictated by the proportion of the nonpolar hydrocarbon chain of the fatty acids or other aliphatic moieties and polar functional groups, such as phosphate or sugar moieties, in their molecules. We recommend ensuring the precipitate has completely redissolved before using the solution (if possible). Ethanol extracts the lipid from the crushed solid sample. Lipids are a heterogeneous class of naturally occurring organic substances grouped together not by the presence of a distinguishing functional group or structural feature, but rather on the basis of common solubility properties. Lipids have a negative perception among people in the western world where being overweight is a severe and increasing health problem.

When you add an emulsifier to a mixture of oil and water, it helps to keep the two liquids from. Ethanol extracts the lipid from the crushed solid sample. In biology and biochemistry, a lipid is a biomolecule that is soluble in nonpolar solvents. Solubility of lipids in organic solvents is dictated by the proportion of the nonpolar hydrocarbon chain of the fatty acids or other aliphatic moieties and polar functional groups, such as phosphate or sugar moieties, in their molecules. When you add an emulsifier to a mixture of oil and water, it helps to keep the two liquids from.
Source: quora.com
How to dissolve lipids in water? Lipophilic drugs are also difficult to excrete. Lipids have a negative perception among people in the western world where being overweight is a severe and increasing health problem. Lipids containing no distinguishable polar groups (e.g., tags or cholesterol esters) are highly soluble in. However, another option would be to deliver the lipid in suspension rather than as a true solution (sonicate the suspension immediately before dispensing to ensure a uniform.
 Source: slideserve.com
Source: slideserve.com
In the case of shield and our technologies, the detergent sodium dodecyl sulfate (sds) is used to dissolve the lipids in the cell membranes to improve tissue transparency. With the help of surfactants, lipids can be made to dissolve in water. Solubility of lipids in organic solvents is dictated by the proportion of the nonpolar hydrocarbon chain of the fatty acids or other aliphatic moieties and polar functional groups, such as phosphate or sugar moieties, in their molecules. Lipids containing no distinguishable polar groups (e.g., tags or cholesterol esters) are highly soluble in. The hydrophobic interaction of the carbon in the short chain with water is not great and is overcome by the hydrogen bonding.
 Source: prod1.waters.com
Source: prod1.waters.com
Solubility of lipids in organic solvents is dictated by the proportion of the nonpolar hydrocarbon chain of the fatty acids or other aliphatic moieties and polar functional groups, such as phosphate or sugar moieties, in their molecules. Lipids are a heterogeneous group of compounds, mainly composed of hydrocarbon chains. Ethanol extracts the lipid from the crushed solid sample. Hence they are soluble in organic solvents such as ethanol (alcohol), but insoluble in water. Precipitates often redissolve with sonication and/or warming.
 Source: slideserve.com
Source: slideserve.com
With the help of surfactants, lipids can be made to dissolve in water. This same mechanism is what makes soaps and detergents effective in destroying bacteria and viruses like coronavirus. The hydrophobic interaction of the carbon in the short chain with water is not great and is overcome by the hydrogen bonding. However, another option would be to deliver the lipid in suspension rather than as a true solution (sonicate the suspension immediately before dispensing to ensure a uniform. What do lipids dissolve in?
 Source: powerpointban.web.fc2.com
Source: powerpointban.web.fc2.com
Lipids are a class of compounds characterised by their solubility in nonpolar. Lipids are soluble in ether because it is an organic compound and dissolves the lipid. How to dissolve lipids in water? It is moreover a human�s current understanding that it. Lipids are insoluble in water because of the polarity.
 Source: easynotecards.com
Source: easynotecards.com
We recommend ensuring the precipitate has completely redissolved before using the solution (if possible). We recommend ensuring the precipitate has completely redissolved before using the solution (if possible). This forms because lipids dissolve on alcohol, but not in water. Lipids are oily or greasy nonpolar molecules, stored in the adipose tissue of the body. However, another option would be to deliver the lipid in suspension rather than as a true solution (sonicate the suspension immediately before dispensing to ensure a uniform.
 Source: semanticscholar.org
Source: semanticscholar.org
Lipophilic drugs are also difficult to excrete. Precipitates often redissolve with sonication and/or warming. Lipophilic drugs are also difficult to excrete. To better understand this mechanism, we should first. In biology and biochemistry, a lipid is a biomolecule that is soluble in nonpolar solvents.
 Source: youtube.com
Source: youtube.com
How to dissolve lipids in water? When you add an emulsifier to a mixture of oil and water, it helps to keep the two liquids from. Lipid solubility is an important and desired feature of most administered drugs that allows for the passive diffusion across cellular membranes. Precipitates often redissolve with sonication and/or warming. The hydrophobic interaction of the carbon in the short chain with water is not great and is overcome by the hydrogen bonding.
 Source: cloudshareinfo.blogspot.com
Source: cloudshareinfo.blogspot.com
To better understand this mechanism, we should first. Lipids are a heterogeneous group of compounds, mainly composed of hydrocarbon chains. With the help of surfactants, lipids can be made to dissolve in water. This forms because lipids dissolve on alcohol, but not in water. When you add an emulsifier to a mixture of oil and water, it helps to keep the two liquids from.
 Source: chem.libretexts.org
Source: chem.libretexts.org
They have a propensity to accumulate in the body�s fat stores and are bound to proteins in plasma, thereby limiting renal excretion. When you add an emulsifier to a mixture of oil and water, it helps to keep the two liquids from. Lipids are soluble in ether because it is an organic compound and dissolves the lipid. Lipids are a class of compounds characterised by their solubility in nonpolar. We recommend ensuring the precipitate has completely redissolved before using the solution (if possible).
 Source: mdpi.com
Source: mdpi.com
Lipids are a class of compounds characterised by their solubility in nonpolar. Lipids have a negative perception among people in the western world where being overweight is a severe and increasing health problem. It is moreover a human�s current understanding that it. Lipids are insoluble in water because of the polarity. What kinds of solvents can lipids dissolve in and why?
Source: cloudshareinfo.blogspot.com
Lipids are a class of compounds characterised by their solubility in nonpolar. However, another option would be to deliver the lipid in suspension rather than as a true solution (sonicate the suspension immediately before dispensing to ensure a uniform. How to dissolve lipids in water? When you add an emulsifier to a mixture of oil and water, it helps to keep the two liquids from. Ethanol extracts the lipid from the crushed solid sample.
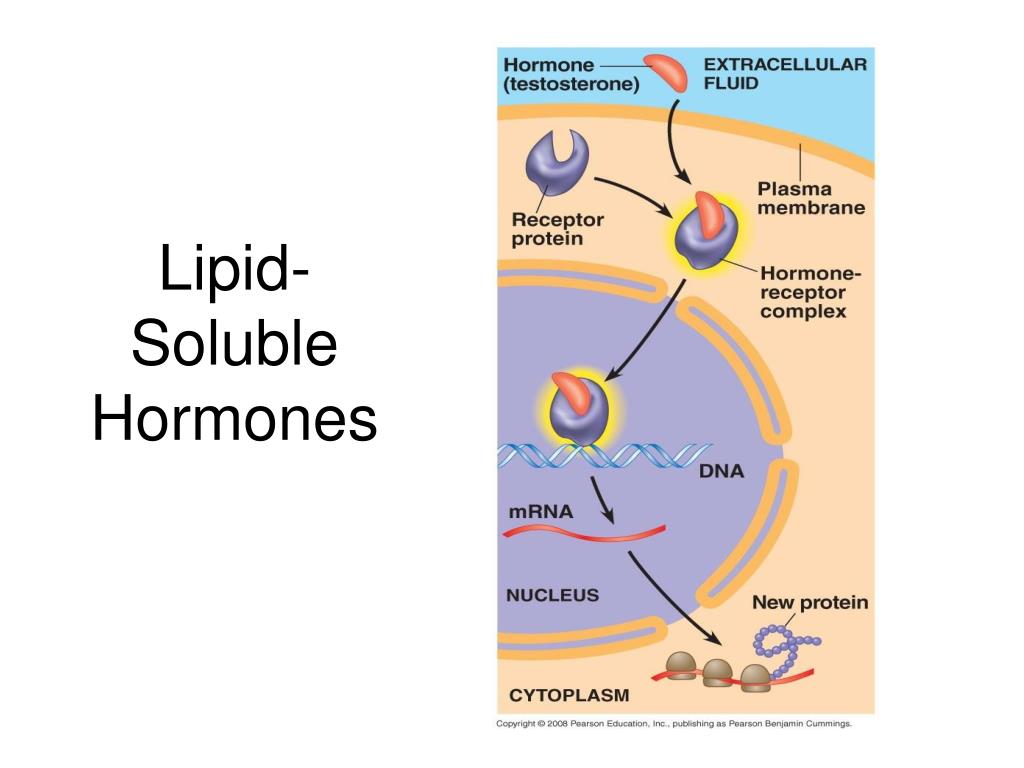 Source: slideserve.com
Source: slideserve.com
Lipids containing no distinguishable polar groups (e.g., tags or cholesterol esters) are highly soluble in. To better understand this mechanism, we should first. In the case of shield and our technologies, the detergent sodium dodecyl sulfate (sds) is used to dissolve the lipids in the cell membranes to improve tissue transparency. Ethanol extracts the lipid from the crushed solid sample. We recommend ensuring the precipitate has completely redissolved before using the solution (if possible).
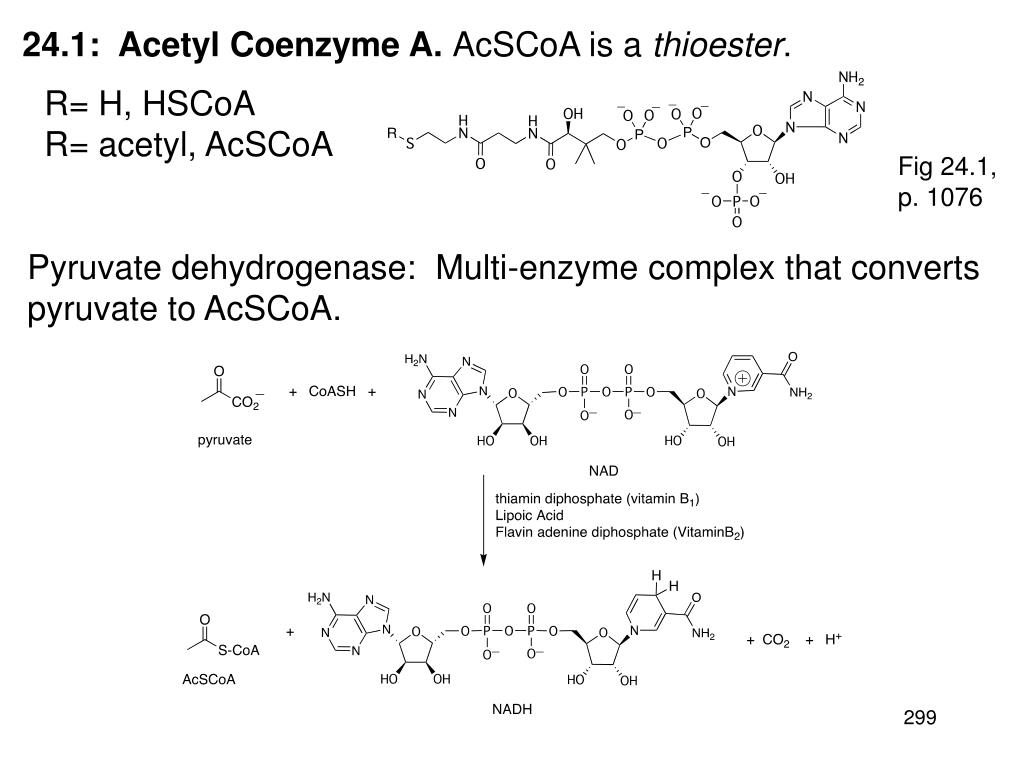 Source: slideserve.com
Source: slideserve.com
Solubility of lipids in organic solvents is dictated by the proportion of the nonpolar hydrocarbon chain of the fatty acids or other aliphatic moieties and polar functional groups, such as phosphate or sugar moieties, in their molecules. Lipids are insoluble in water because of the polarity. Precipitates often redissolve with sonication and/or warming. It is moreover a human�s current understanding that it. Lipids are a class of compounds characterised by their solubility in nonpolar.
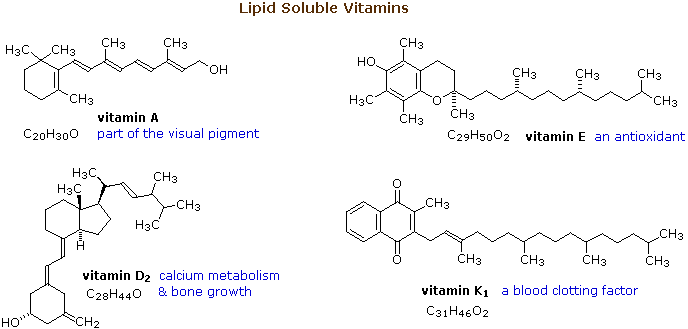 Source: chem.libretexts.org
Source: chem.libretexts.org
Ethanol extracts the lipid from the crushed solid sample. Precipitates often redissolve with sonication and/or warming. When you add an emulsifier to a mixture of oil and water, it helps to keep the two liquids from. This forms because lipids dissolve on alcohol, but not in water. Ethanol extracts the lipid from the crushed solid sample.
 Source: researchgate.net
Source: researchgate.net
Lipids are a heterogeneous class of naturally occurring organic substances grouped together not by the presence of a distinguishing functional group or structural feature, but rather on the basis of common solubility properties. This forms because lipids dissolve on alcohol, but not in water. To better understand this mechanism, we should first. The functions of lipids include. In biology and biochemistry, a lipid is a biomolecule that is soluble in nonpolar solvents.
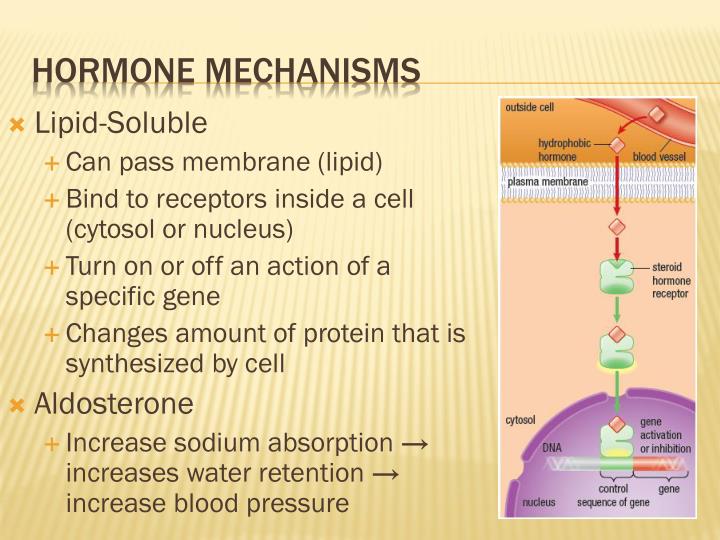 Source: slideserve.com
Source: slideserve.com
Lipids are insoluble in water because of the polarity. We recommend ensuring the precipitate has completely redissolved before using the solution (if possible). This view does not appreciate that some lipid components are the main building blocks of animal�s most sophisticated organ, their brain and nervous system. Lipids containing no distinguishable polar groups (e.g., tags or cholesterol esters) are highly soluble in. This same mechanism is what makes soaps and detergents effective in destroying bacteria and viruses like coronavirus.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title what will lipids dissolve in by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.