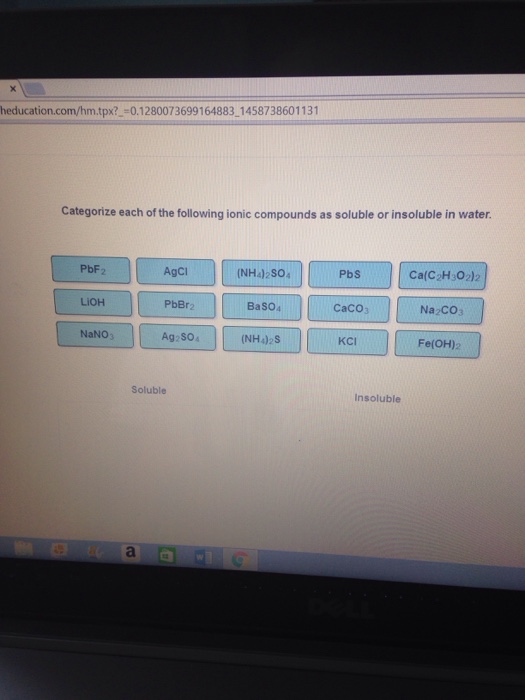
Which of the following compounds is soluble in water
Which Of The Following Compounds Is Soluble In Water. For example, sugar is a polar compound, and it is dissolved in water at 80°c. Which of the following compounds is water soluble? Which of the following compound are the soluble in water? Ion compounds ion compounds are compounds that are created from atoms that share an ionic bond, where electrons are.
 Which of the following compounds are readily soluble in water? YouTube From youtube.com
Which of the following compounds are readily soluble in water? YouTube From youtube.com
Select the correct answer below: Complete and balance each of the following: So we have to find out which among the following are soluble in water. Chromic oxide, and m os2, and h gi 2 are all as soluble as bricks in aqueous phases. But there are some exceptions to this rule. Which of the following compounds will be more soluble in acidic solution than in pure water?!
Which of the following compounds is water soluble?
The ionic compound that is insoluble in water is sugar. Due to the lack of strong interaction with water its solubility in water is very less (or not soluble). Chromic oxide, and m os2, and h gi 2 are all as soluble as bricks in aqueous phases. Which of the following compounds is water soluble? Which of the following compounds is soluble in water? As ionic compounds are soluble in water and covalent compounds are insoluble in water, nacl is soluble in water.
 Source: youtube.com
Source: youtube.com
Here are few inorganic salts given. I will recap the most common solubility rules and i will mention specific compounds that are. Complete and balance each of the following: Select the correct answer below: Chromic oxide, and m os2, and h gi 2 are all as soluble as bricks in aqueous phases.
 Source: chegg.com
Source: chegg.com
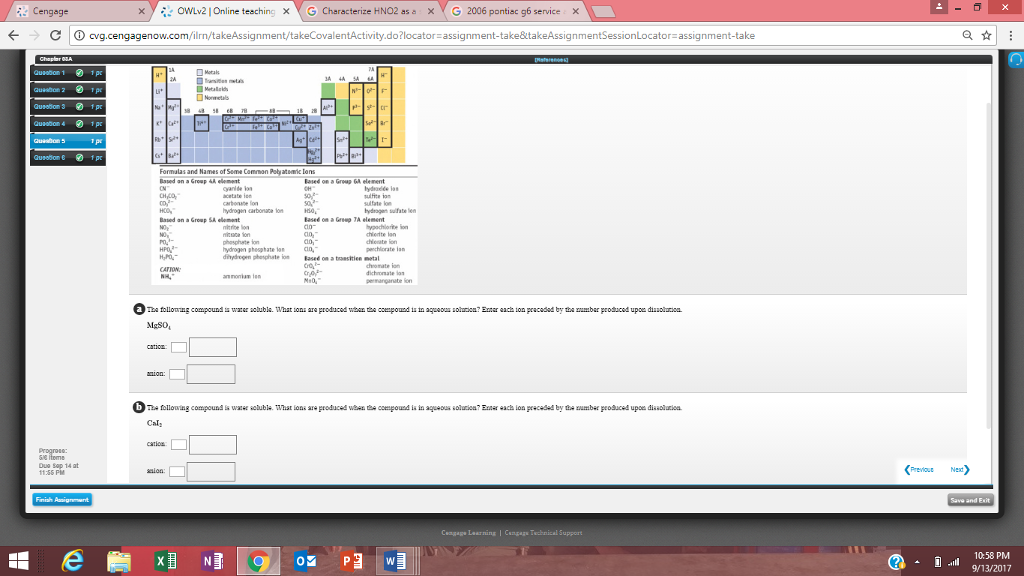
A) pbcl2 => all chlorides and bromides and iodides are soluble except pb+ , ag+ , hg2 2+ => insoluble b) pb (no3)2 => all nitrates are. Which of the following compounds is water soluble? The reason is due to the magnitude of lattice energy remains almost constant as the sulphate is so big that small increase in the size of the cation from be to ba does not make any difference. Solubility is the ability of a solid, liquid, or gaseous chemical substance (referred to as the solute) to dissolve in solvent (usually a liquid) and form a solution. O cul o pbcl2 ca(cio), o fes o none of the above will be more soluble in acidic solution.
As an example, methane is soluble in ethanol, diethyl ether, benzene, toluene, methanol, acetone. Due to the lack of strong interaction with water its solubility in water is very less (or not soluble). As an example, methane is soluble in ethanol, diethyl ether, benzene, toluene, methanol, acetone. But it cannot make any strong bond (like hydrogen bond) with water. Its solubility is the most important property.
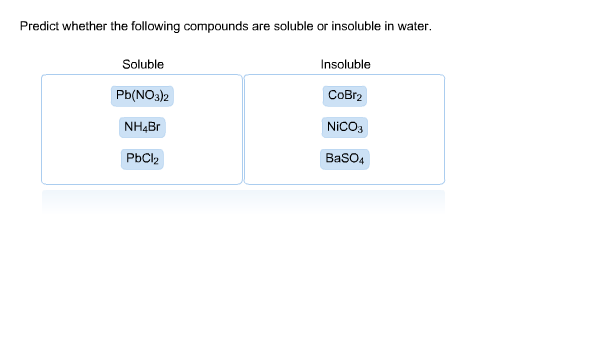 Source: chegg.com
Source: chegg.com
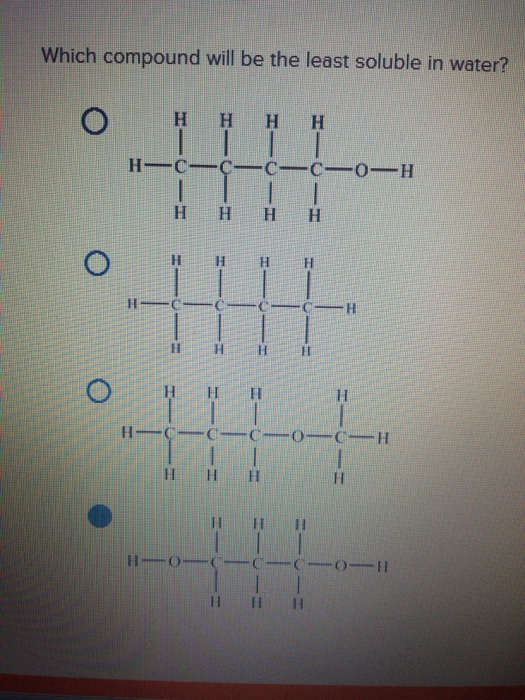
So we have to find out which among the following are soluble in water. Which of the following compounds is most soluble? The solubility product increases as the temperature of the solvent increases. This question has multiple correct options. Solubility can be defined as the maximum amount of a solute that can dissolve in a liquid.

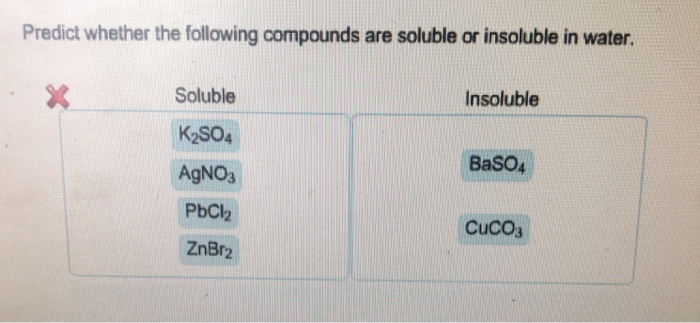
The ionic compound that is insoluble in water is sugar. So we have to find out which among the following are soluble in water. O cul o pbcl2 ca(cio), o fes o none of the above will be more soluble in acidic solution. So let us go to the answer. Indicate if each of the following compounds is soluble in water.
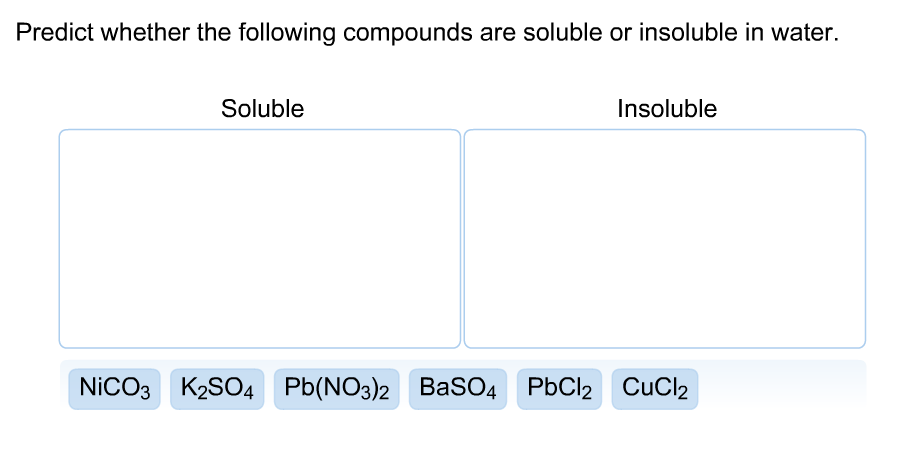 Source: chegg.com
Source: chegg.com
This question has multiple correct options. Mgso4 + k3po4 in which of the following pairs of ionic compounds are both chemicals soluble in water? Its solubility is the most important property. Which of the following compounds is soluble in water? Sugar is one of the most soluble compounds in water.
 Source: youtube.com
Source: youtube.com
Which of the following compounds is most soluble? The reason is due to the magnitude of lattice energy remains almost constant as the sulphate is so big that small increase in the size of the cation from be to ba does not make any difference. Ion compounds ion compounds are compounds that are created from atoms that share an ionic bond, where electrons are. But insoluble in water (22.7 mg of ch 4 / 1 litre of water) The ionic compound that is insoluble in water is sugar.
 Source: youtube.com
Source: youtube.com
The correct option is a nacl. Complete and balance each of the following: Ascl3(l) +3h 2o(l) → as(oh)3(aq) + 3h cl(aq) p b(n o3)2 speciates in aqueous solution to give stoichiometric p b2. Which of the following compounds is most soluble? As an example, methane is soluble in ethanol, diethyl ether, benzene, toluene, methanol, acetone.
 Source: chegg.com
Source: chegg.com
It will dissolve in hotter water. They do not dissolve in water. Ion compounds ion compounds are compounds that are created from atoms that share an ionic bond, where electrons are. It will dissolve in hotter water. So in option a we have zidane and so forth, zinc sulfate is a colorless soil when added to water.
Ascl3(l) +3h 2o(l) → as(oh)3(aq) + 3h cl(aq) p b(n o3)2 speciates in aqueous solution to give stoichiometric p b2. The ionic compound that is insoluble in water is sugar. Study exercise 3 name write chemical formulas for the following compounds: So let us go to the answer. I will recap the most common solubility rules and i will mention specific compounds that are.
 Source: youtube.com
Source: youtube.com
Due to the lack of strong interaction with water its solubility in water is very less (or not soluble). Select the correct answer below: In which of the following pairs of ionic compounds are both chemicals soluble in water? So we have to find out which among the following are soluble in water. Ion compounds ion compounds are compounds that are created from atoms that share an ionic bond, where electrons are.
 Source: chegg.com
Source: chegg.com
Its solubility is the most important property. Mgso4 + k3po4 in which of the following pairs of ionic compounds are both chemicals soluble in water? A) pbcl2 => all chlorides and bromides and iodides are soluble except pb+ , ag+ , hg2 2+ => insoluble b) pb (no3)2 => all nitrates are. O cul o pbcl2 ca(cio), o fes o none of the above will be more soluble in acidic solution. Which of the following compound are the soluble in water?
 Source: youtube.com
Source: youtube.com
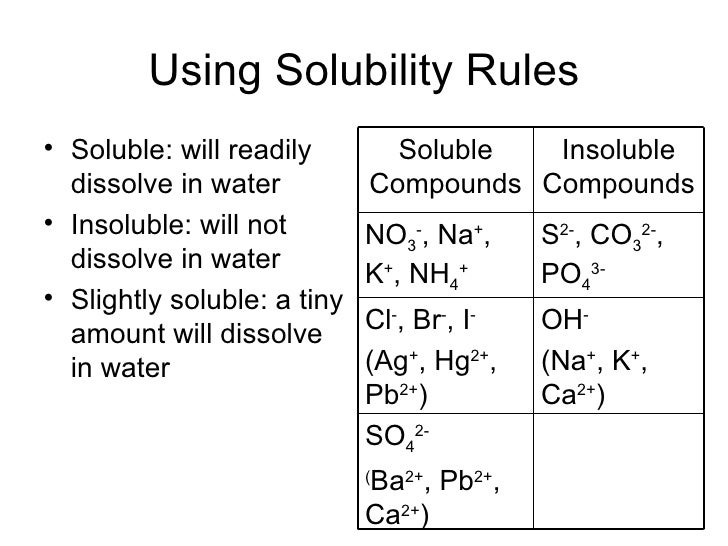
Solubility is the ability of a solid, liquid, or gaseous chemical substance (referred to as the solute) to dissolve in solvent (usually a liquid) and form a solution. But there are some exceptions to this rule. O cul o pbcl2 ca(cio), o fes o none of the above will be more soluble in acidic solution. Hydration energy decreases down the group,so solubility with water decreases down the group.therefore caf 2 is highly soluble in water. The following sections discuss both soluble and insoluble compounds in water.
![[Solved] Arrange the following compounds in order of increasing [Solved] Arrange the following compounds in order of increasing](https://examquiz.netlify.app/img/placeholder.svg)
Solubility can be defined as the maximum amount of a solute that can dissolve in a liquid. Drag the appropriate items to their respective bins. Which of the following compounds is soluble in water? O cul o pbcl2 ca(cio), o fes o none of the above will be more soluble in acidic solution. The following sections discuss both soluble and insoluble compounds in water.
 Source: slideshare.net
Source: slideshare.net
As ionic compounds are soluble in water and covalent compounds are insoluble in water, nacl is soluble in water. So in option a we have zidane and so forth, zinc sulfate is a colorless soil when added to water. Solubility can be defined as the maximum amount of a solute that can dissolve in a liquid. The solubility product increases as the temperature of the solvent increases. caf_(2) is least soluble in among other fluori asked jan 28 in chemistry by prathamsingh ( 25.0k points)
 Source: chegg.com
Source: chegg.com
But it cannot make any strong bond (like hydrogen bond) with water. What is a soluble compound? The ionic compound that is insoluble in water is sugar. But insoluble in water (22.7 mg of ch 4 / 1 litre of water) Some organic compounds are soluble only in organic solvents.
 Source: socratic.org
Source: socratic.org
Hydration energy decreases down the group,so solubility with water decreases down the group.therefore caf 2 is highly soluble in water. Solubility can be defined as the maximum amount of a solute that can dissolve in a liquid. So in option a we have zidane and so forth, zinc sulfate is a colorless soil when added to water. Hydration energy decreases down the group,so solubility with water decreases down the group.therefore caf 2 is highly soluble in water. Indicate if each of the following compounds is soluble in water.
 Source: homeworklib.com
Source: homeworklib.com
Ascl3(l) +3h 2o(l) → as(oh)3(aq) + 3h cl(aq) p b(n o3)2 speciates in aqueous solution to give stoichiometric p b2. So in option a we have zidane and so forth, zinc sulfate is a colorless soil when added to water. Mgso4 + k3po4 in which of the following pairs of ionic compounds are both chemicals soluble in water? Thus beso 4 and mgso 4 are highly soluble, caso4 is sparingly soluble but the sulphates of sr,ba and ra are insoluble in water. It will dissolve in hotter water.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title which of the following compounds is soluble in water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.