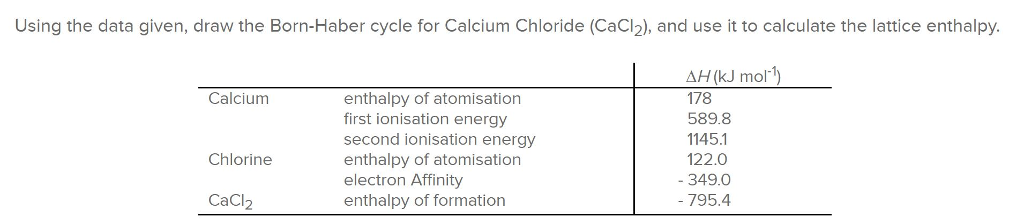
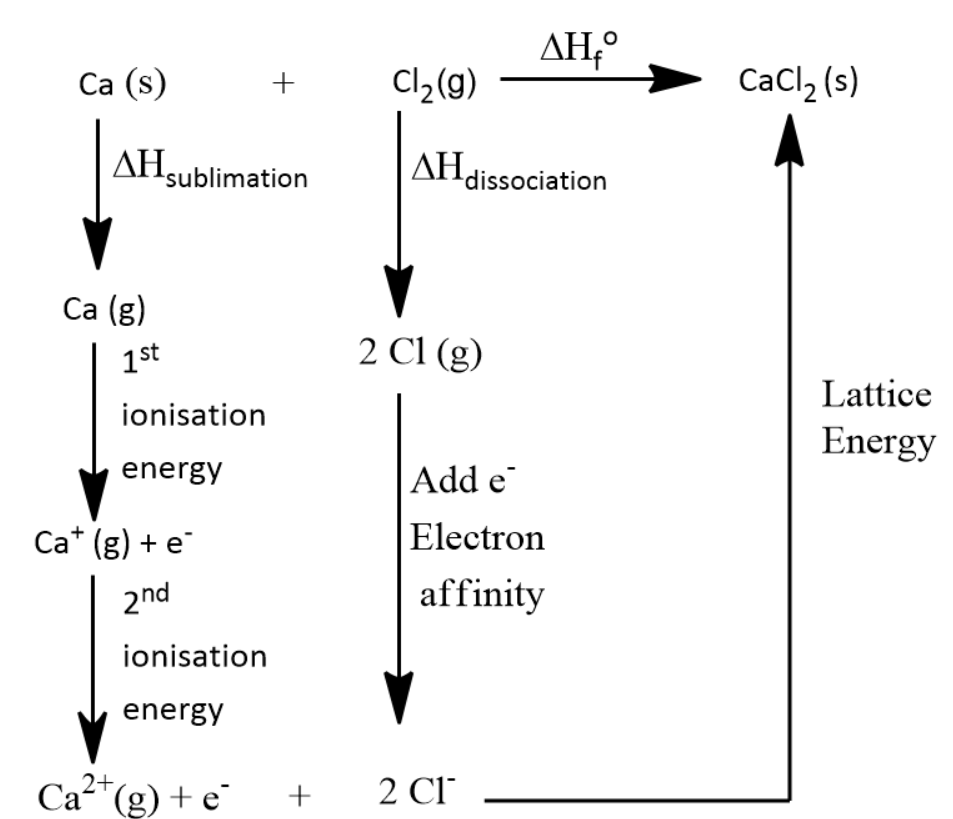
Calculate the lattice energy of calcium chloride
Calculate The Lattice Energy Of Calcium Chloride. Lattice energy can be defined as the energy required to convert one mole of an ionic solid into gaseous ionic constituents. So we�re calculating the lattice energy of calcium chloride. Ca(s) + cl 2 (g) cacl 2 (s) Determine the lattice energy by subtracting steps 2 & 3 from step 1.
 Solved Using The Data Given, Draw The BornHaber Cycle Fo… From chegg.com
Solved Using The Data Given, Draw The BornHaber Cycle Fo… From chegg.com
Energy needed to vaporize one mole of ca(s) is 192kj. Expert qa ask unlimited questions and get expert help right away. Answer of using the following data, calculate the lattice energy of calcium chloride: To calculate the enthalpy change from solid. For calcium the first ionization energy is 589.5kj/mol and the second ionization energy is 1146kj/mol. Calculate the lattice energy for cacl2 from the following information:
The science, which deals with crystals properties is crystallography.
Ca(s) + cl 2 (g) cacl 2 (s) Lattice energy can be defined as the energy required to convert one mole of an ionic solid into gaseous ionic constituents. We can again use the born harbor cycle. Expert qa ask unlimited questions and get expert help right away. Cas cl 2 g cacl 2 s 2 iii use your answer to ii and the following data together with relevant data from the data booklet to calculate a value for for calcium chloride. Get the answers you need, now!

Expert qa ask unlimited questions and get expert help right away. Lattice energy can be defined as the energy required to convert one mole of an ionic solid into gaseous ionic constituents. So we�re calculating the lattice energy of calcium chloride. So we�re calculating the lattice energy of calcium chloride. Ca(s) + cl 2 (g) cacl 2 (s)
 Source: fin3tutor.blogspot.com
Source: fin3tutor.blogspot.com
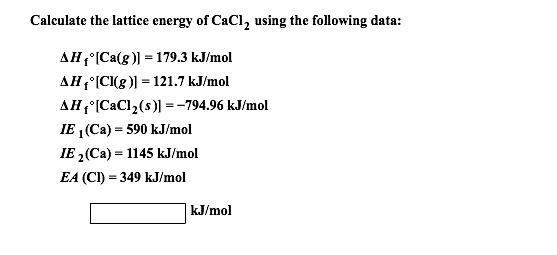
(see table 8.2 and 8.3 for other data.) %3d. For calcium the first ionization energy is 589.5kj/mol and the second ionization energy is 1146kj/mol. 2 (a) calcium metal reacts with chlorine gas to form calcium chloride, cacl 2. This is because the magnitude of the positive charge. Doortree8387 doortree8387 01/30/2020 chemistry college answered calculate the lattice energy of calcium chloride given that the heat of sublimation of ca is 121 kj/mol and ¢h°f(cacl2) 5 2795 kj/mol.
 Source: yeahchemistry.com
Source: yeahchemistry.com
Ca(s) + cl 2 (g) cacl 2 (s) For calcium the first ionization energy is 589.5kj/mol and the second ionization energy is 1146kj/mol. Ca(s) + cl 2 (g) cacl 2 (s) Calculate the lattice energy of calcium chloride given that the heat of sublimation of ca is 121 kj/mol and. This is because the magnitude of the positive charge.
 Source: chegg.com
Source: chegg.com
So we�re calculating the lattice energy of calcium chloride. Energy needed to dissociate 1/2 mole of cl2 into cl atoms = 121.4. The lattice energy is usually given in kilojules per mole (kj/mol). So we have calcium in the solid, which is elemental state going to calcium in the gas phase, we have calcium in the gas phase, going to. We can again use the born harbor cycle.
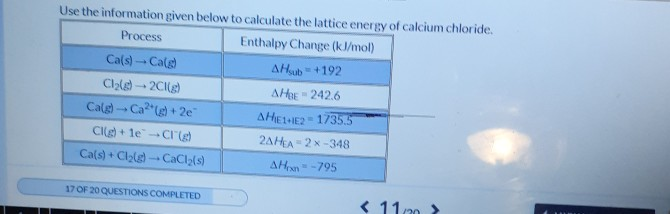 Source: chegg.com
Source: chegg.com
For example, the lattice energy of calcium chloride is greater than that of potassium chloride despite the similarity in the crystal arrangements of these compounds. Calcium chloride is an essential salt of calcium that has many applications for household and industrial use. For example, the lattice energy of calcium chloride is greater than that of potassium chloride despite the similarity in the crystal arrangements of these compounds. Determine the lattice energy by subtracting steps 2 & 3 from step 1. We can again use the born harbor cycle.
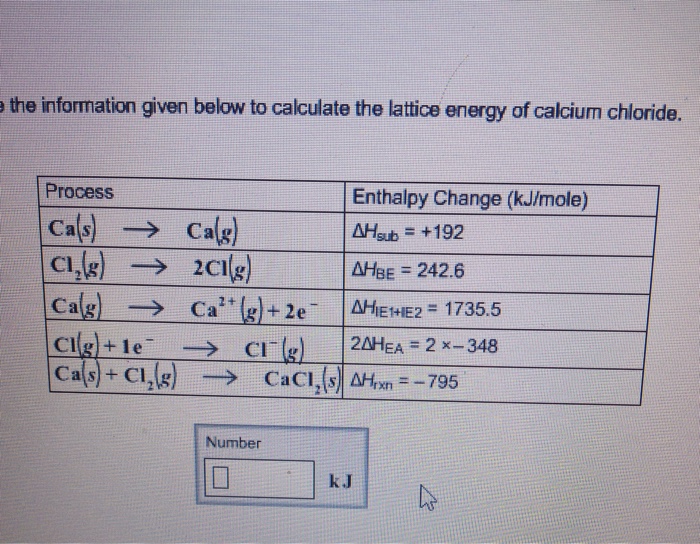
Lattice energy can be defined as the energy required to convert one mole of an ionic solid into gaseous ionic constituents. Cas cl 2 g cacl 2 s 2 iii use your answer to ii and the following data together with relevant data from the data booklet to calculate a value for for calcium chloride. (i) write an equation, including state symbols, to represent the lattice energy of calcium chloride, cacl 2. So we�re calculating the lattice energy of calcium chloride. So i suppose in this question lattice energy is the opposite.

The lattice energy of solid n a c l is 1 8 0 k c a l m o l − 1. Cacl 2 is the chemical calcium chloride formula and its molar mass is 110,983 g/mol. Calculate the lattice energy of calcium chloride given that the heat of sublimation of ca is 121 kj/mol and. The calcium cation (ca 2+) and two chlorine anions are an ionic compound (cl. So we�re calculating the lattice energy of calcium chloride.
 Source: vedantu.com
Source: vedantu.com
Calculate the lattice energy of calcium chloride. 2 (a) calcium metal reacts with chlorine gas to form calcium chloride, cacl 2. Use the information given below to calculate the lattice energy of calcium chloride. Use hess’s law to calculate the lattice energy of calcium chloride. Calcium chloride is an essential salt of calcium that has many applications for household and industrial use.
 Source: learnah.org
Source: learnah.org
Cesium to l uorine, chlorine to chlorine, bromine to chlorine, silicon to carbon. .26 calculate the lattice energy of calcium chloride given that the heat of sublimation of ca is 121 kj/mol and dh°f (cacl2) 5 2795 kj/mol. Use hess’s law to calculate the lattice energy of calcium chloride. Ca(s) + cl 2 (g) cacl 2 (s) Calculate the lattice energy for cacl2 from the following information:
 Source: tessshebaylo.com
Get the answers you need, now! Using the following data, calculate the lattice energy of calcium chloride: So i suppose in this question lattice energy is the opposite. The calcium cation (ca 2+) and two chlorine anions are an ionic compound (cl. Doortree8387 doortree8387 01/30/2020 chemistry college answered calculate the lattice energy of calcium chloride given that the heat of sublimation of ca is 121 kj/mol and ¢h°f(cacl2) 5 2795 kj/mol.
 Source: vedantu.com
Source: vedantu.com
The dissolution of the solid in water in the form of ions is endothermic to the extent of 1 k c a l m o l − 1. Energy needed to dissociate 1/2 mole of cl2 into cl atoms = 121.4. The lattice energy is usually given in kilojules per mole (kj/mol). The calcium cation (ca 2+) and two chlorine anions are an ionic compound (cl. (i) write an equation, including state symbols, to represent the lattice energy of calcium chloride, cacl 2.

So we�re calculating the lattice energy of calcium chloride. So we�re calculating the lattice energy of calcium chloride. Cacl 2 is the chemical calcium chloride formula and its molar mass is 110,983 g/mol. Lattice energy can be defined as the energy required to convert one mole of an ionic solid into gaseous ionic constituents. (see table 8.2 and 8.3 for other data.) %3d.
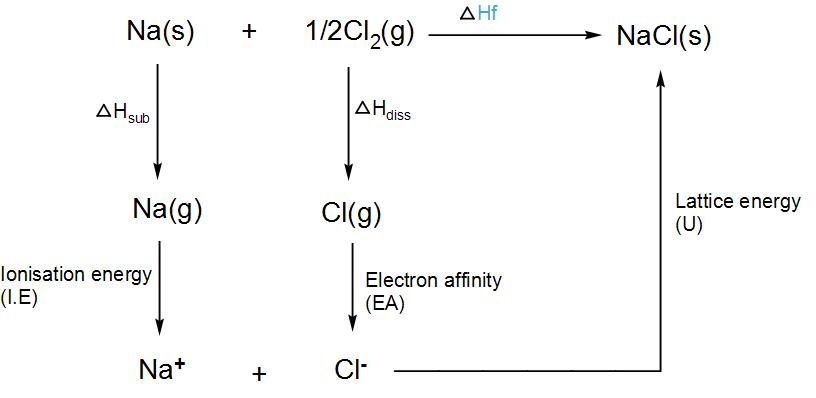
![New Page 2 [www.chemhume.co.uk]](http://www.chemhume.co.uk/A2CHEM/Unit 2b/9 Lattice enthalpy/born-haber_sodium_chloride.jpg “New Page 2 [www.chemhume.co.uk]") Source: chemhume.co.uk
Energy needed to dissociate 1/2 mole of cl2 into cl atoms = 121.4. Calculate the lattice energy of calcium chloride. The lattice energy is usually given in kilojules per mole (kj/mol). The calcium cation (ca 2+) and two chlorine anions are an ionic compound (cl. Using the following data, calculate the lattice energy of calcium chloride:
 Source: brainly.in
Source: brainly.in
Lattice energy can be defined as the energy required to convert one mole of an ionic solid into gaseous ionic constituents. This is because the magnitude of the positive charge. (see table 8.2 and 8.3 for other data.) %3d. Doortree8387 doortree8387 01/30/2020 chemistry college answered calculate the lattice energy of calcium chloride given that the heat of sublimation of ca is 121 kj/mol and ¢h°f(cacl2) 5 2795 kj/mol. Calcium chloride is an essential salt of calcium that has many applications for household and industrial use.
 Source: chegg.com
Source: chegg.com
Usually the lattice energy is the change in enthalpy for the formation of a lattice from ions and this produces a negative energy. For example, the lattice energy of calcium chloride is greater than that of potassium chloride despite the similarity in the crystal arrangements of these compounds. Cesium to l uorine, chlorine to chlorine, bromine to chlorine, silicon to carbon. Use hess’s law to calculate the lattice energy of calcium chloride. Lattice energy can be defined as the energy required to convert one mole of an ionic solid into gaseous ionic constituents.
 Source: sharedocnow.blogspot.com
Source: sharedocnow.blogspot.com
So we have calcium in the solid, which is elemental state going to calcium in the gas phase, we have calcium in the gas phase, going to. The lattice energy is the total potential energy of the crystal. So we have calcium in the solid, which is elemental state going to calcium in the gas phase, we have calcium in the gas phase, going to. We can again use the born harbor cycle. Calculate the lattice energy for cacl2 from the following information:
 Source: chegg.com
Source: chegg.com
We can again use the born harbor cycle. Using the following data, calculate the lattice energy of calcium chloride: .26 calculate the lattice energy of calcium chloride given that the heat of sublimation of ca is 121 kj/mol and dh°f (cacl2) 5 2795 kj/mol. Calcium chloride is an essential salt of calcium that has many applications for household and industrial use. Determine the lattice energy by subtracting steps 2 & 3 from step 1.

The dissolution of the solid in water in the form of ions is endothermic to the extent of 1 k c a l m o l − 1. The lattice energy is the total potential energy of the crystal. If the hydration energies of n a + and c l − are in the ratio 6:5, then the enthalpy of hydration of n a + ion is: Science chemistry q&a library 4. The lattice energy of solid n a c l is 1 8 0 k c a l m o l − 1.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title calculate the lattice energy of calcium chloride by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.