Determining the formula of a hydrate
Determining The Formula Of A Hydrate. After heating, the mass of the anhydrous compound is found to be 3.22 g. Remember that a hydrate is an ionic compound which has a certain number of molecules in the spaces of the salt crystal between the ions. Find the formula and name of the hydrate. Determine the formula of the hydrate and then write out the name of the hydrate.
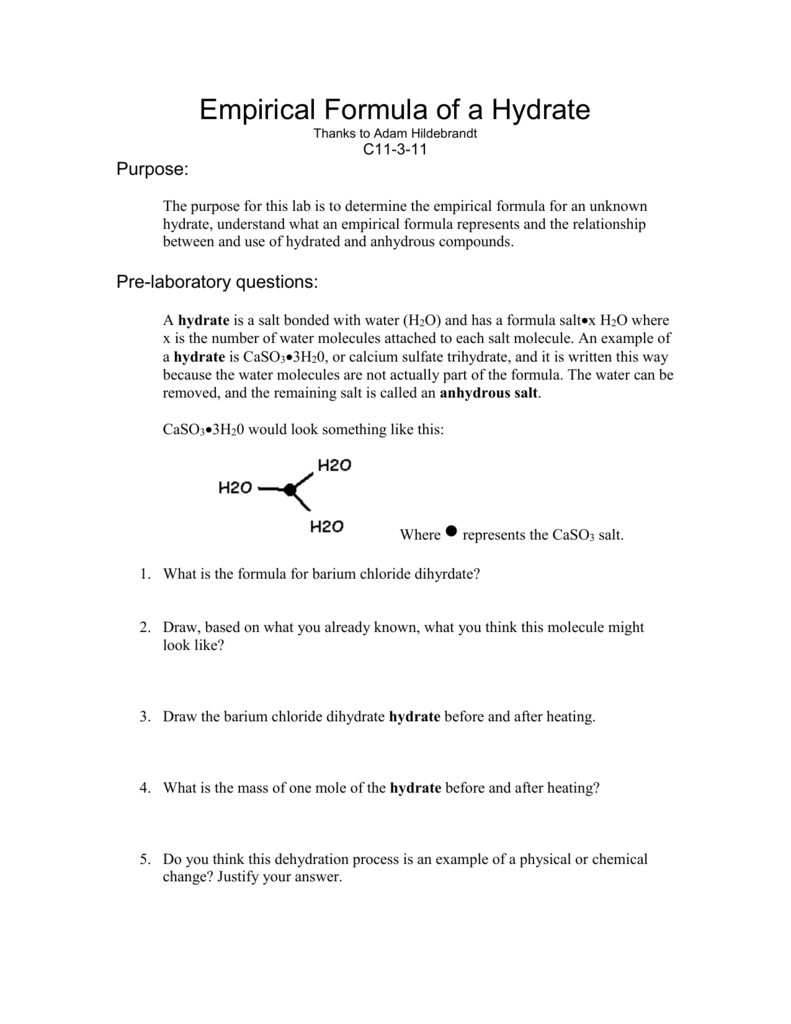 Empirical Formula of a Hydrate From studylib.net
Empirical Formula of a Hydrate From studylib.net
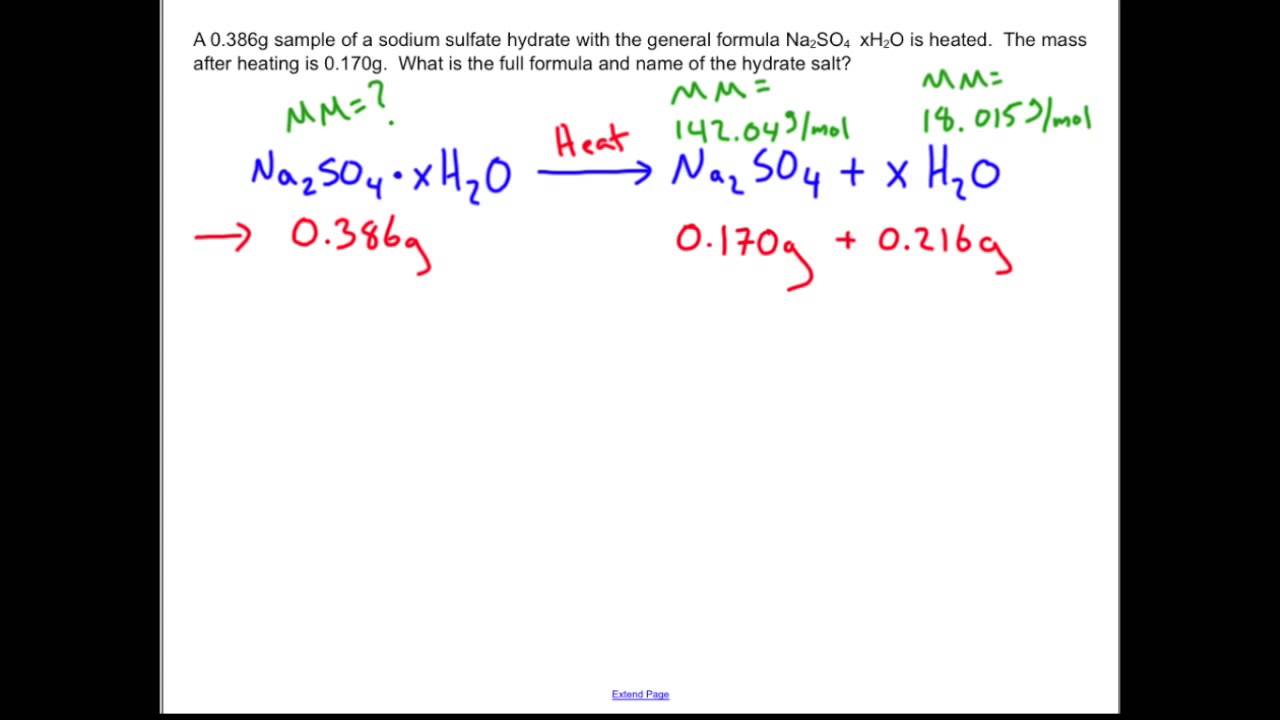
Determining the percentage of water and the chemical formula of a hydrate. 3.52 g ⋅ 1 molebacl2 208.2 g = 0.017 moles. Water has a polar structure and it has positively and negatively charged parts within each molecule. Interestingly, it is common for the hydrated form of a compound to be of a different color than the anhydrous form, which has no water in its structure. A hydrate of na2co3 has a mass of 4.31 g before heating. To determine the chemical formula of cuso4 • xh2o.
When you react 3.9267 grams of na2co3 · nh2o with excess hcl(aq.
To name a hydrate, we name the ionic compound followed by a greek prefix to indicate the number of water molecules ending in the word “hydrate.” (these are. Determine the formula for the hydrate. To determine the chemical formula of cuso4 • xh2o. Heat the anhydrous product to a constant mass., e.g., heat znso 4. Remember that a hydrate is an ionic compound which has a certain number of molecules in the spaces of the salt crystal between the ions. I hypothesize that when the solution is heated the hydrate will convert to an anhydrous ionic compound.
 Source: study.com
Source: study.com
An example of a hydrate is cobalt(ii) nitrate hexahydrate, co(no3)2∙6h2o. Determining the empirical formula of a hydrate october 14th, 2017. Ring stand iron ring crucible and crucible lid crucible tongs wire. Heat the anhydrous product to a constant mass., e.g., heat znso 4. A hydrate of na2co3 has a mass of 4.31 g before heating.
 Source: youtube.com
Source: youtube.com
Therefore, the formula for the hydrate of barium. Determining the empirical formula of a hydrate october 14th, 2017. I hypothesize that when the solution is heated the hydrate will convert to an anhydrous ionic compound. Determining the formula for a hydrate means discovering the number of water molecules that the substance contains. To determine the percentage composition of water in a hydrate of copper (ii) sulfate.
 Source: youtube.com
Source: youtube.com
How can i determine the formula of a hydrate? Determine the formula of the hydrate and then write out the name of the hydrate. A hydrate of na2co3 has a mass of 4.31 g before heating. Ionic compounds that contain a transition metal are often highly colored. This gives it a strong attraction toward ions.
 Source: youtube.com
Source: youtube.com
Then the percentage composition will be determined by weighing. Interestingly, it is common for the hydrated form of a compound to be of a different color than the anhydrous form, which has no water in its structure. Procedure (overview) determine the mass of a dry crucible. Calculating amount of water in hydrate. A hydrate of na 2 co 3 has a mass of 4.31 g before heating.
 Source: studylib.net
Source: studylib.net
The formula of a hydrate is represented by the formula of the ionic compound followed by a raised dot and the number of molecules of water attached per unit for example, cuso4 x 5 h2o. How can i determine the formula of a hydrate? Determination of the formula of a hydrate. Therefore, the formula for the hydrate of barium. A sample of copper (il) sulfate hydrate has a mass of 3.97 g.
 Source: slideserve.com
Source: slideserve.com
To determine the percent by mass of water in a hydrate of copper (ii) sulfate hydrate. Ionic compounds that contain a transition metal are often highly colored. I hypothesize that when the solution is heated the hydrate will convert to an anhydrous ionic compound. Determining the empirical formula of a hydrate c general chemistry scc 201 experiment. A hydrate of na2co3 has a mass of 4.31 g before heating.
 Source: slideshare.net
Source: slideshare.net
1 answer ramith hettiarachchi jun 18, 2014 this 10 minute video will help you ! To determine the percent by mass of water in a hydrate of copper (ii) sulfate hydrate. After heating, the mass of the anhydrous compound is found to be 3.22 g. Determine the formula of the hydrate and then write out the name of the hydrate. Heat to vaporize waters of hydration.
 Source: slideserve.com
Source: slideserve.com
When 5.00 g of iron (iii) chloride hydrate are heated, 2.00 g of water are. The formula of the hydrate shows the ratio of the moles of anhydrous salt to the moles of water. A hydrate of magnesium sulfate has a mass of 13.52 g. A sample of copper (il) sulfate hydrate has a mass of 3.97 g. Water has a polar structure and it has positively and negatively charged parts within each molecule.
 Source: slideserve.com
Source: slideserve.com
After heating, the mass of the anhydrous compound is found to be 3.22 g. Then the percentage composition will be determined by weighing. Therefore, the formula for the hydrate of barium. Barium chloride dihydrate (bacl 2 ⋅2 h 2 o) b) equipment: In this experiment you will discover the formula of an unknown hydrate.
 Source: youtube.com
Source: youtube.com
Determining the percentage of water and the chemical formula of a hydrate. After heating, the mass of the anhydrous compound is found to be 3.22 g. When 5.00 g of fec13 xh20 are heated, 2.00 g of h20 are driven off. To name a hydrate, we name the ionic compound followed by a greek prefix to indicate the number of water molecules ending in the word “hydrate.” (these are. Heat the anhydrous product to a constant mass., e.g., heat znso 4.
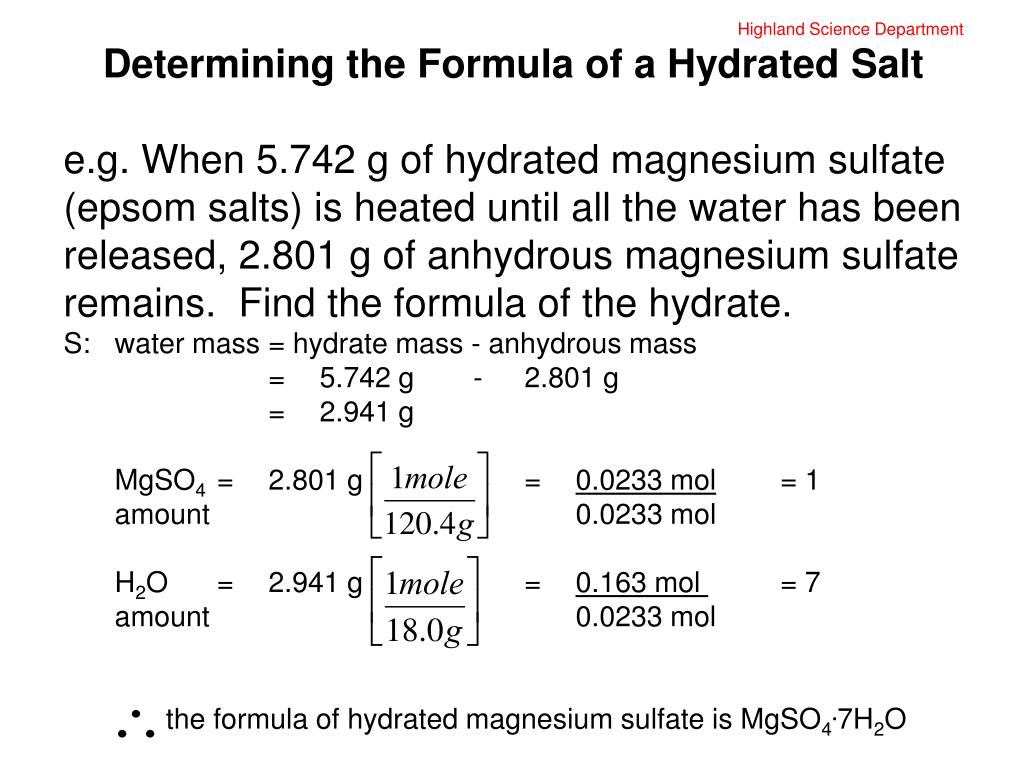 Source: slideserve.com
Source: slideserve.com
If the hydrated copper (ii) sulfate turns to a greyish, white color after being heated on a hot plate with water, then that means the product compound is anhydrous copper (ii) sulfate because the hot plate made the water. Barium chloride dihydrate (bacl 2 ⋅2 h 2 o) b) equipment: In this experiment you will discover the formula of an unknown hydrate. I hypothesize that when the solution is heated the hydrate will convert to an anhydrous ionic compound. A sample of copper (il) sulfate hydrate has a mass of 3.97 g.
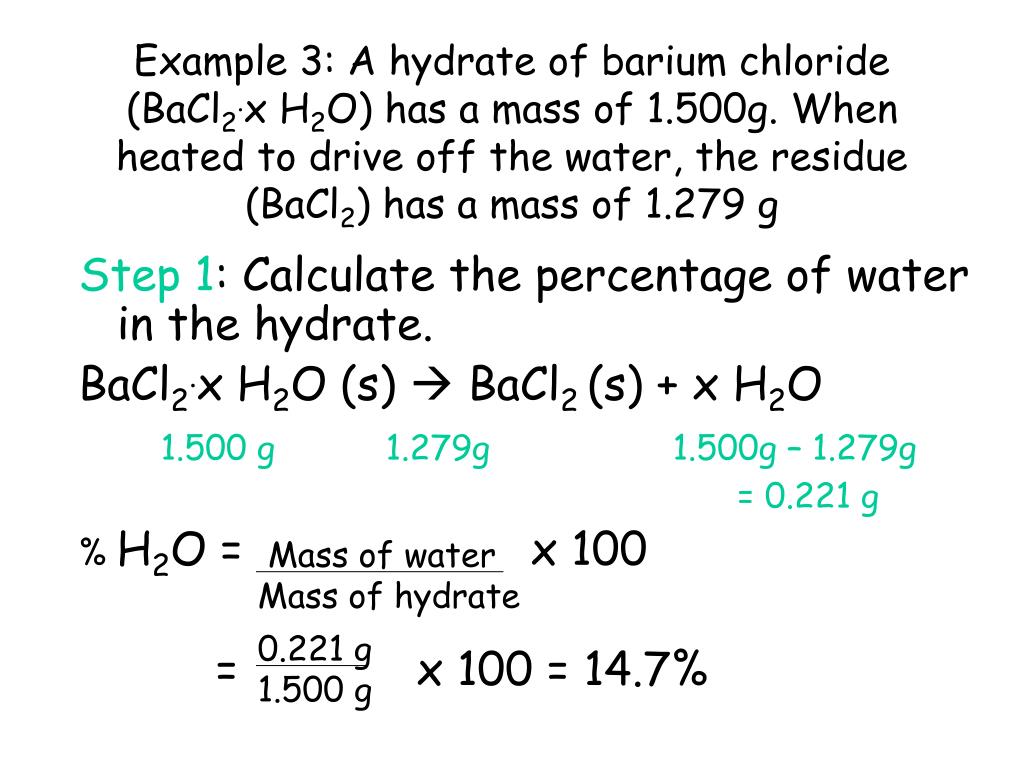 Source: slideserve.com
Source: slideserve.com
Remember that a hydrate is an ionic compound which has a certain number of molecules in the spaces of the salt crystal between the ions. The formula for water is set apart at the end of the formula with a dot, followed by a coefficient that represents the number of water molecules per formula unit. This gives it a strong attraction toward ions. A hydrate is a compound that has one or more water molecules bound to each formula unit. A sample of copper (il) sulfate hydrate has a mass of 3.97 g.

If the hydrated copper (ii) sulfate turns to a greyish, white color after being heated on a hot plate with water, then that means the product compound is anhydrous copper (ii) sulfate because the hot plate made the water. Therefore, the formula for the hydrate of barium. A hydrate of magnesium sulfate has a mass of 13.52 g. What is the formula of the hydrate? Find the chemical formula and the name of the hydrate.
 Source: markedbyteachers.com
Source: markedbyteachers.com
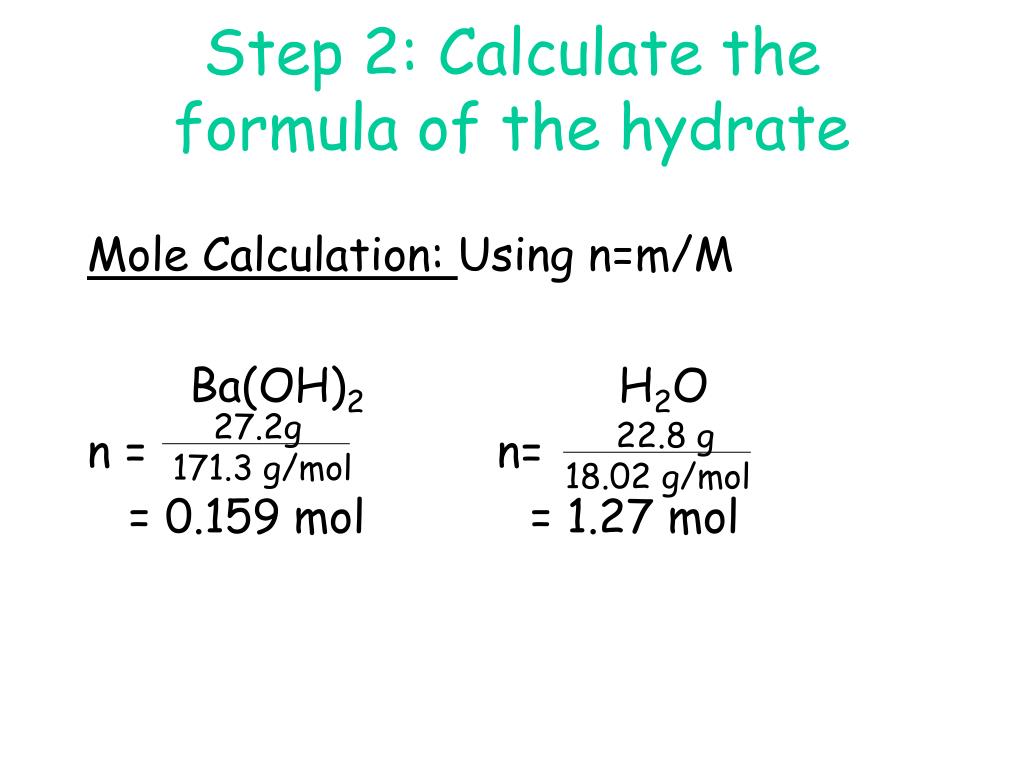
To determine the chemical formula of cuso4 • xh2o. Moles of water moles of anhydrate = 0.034 0.017 = 2. When 5.00 g of iron (iii) chloride hydrate are heated, 2.00 g of water are. You now go on to determine the moles of the anhydrous salt, bacl2. Find the chemical formula and the name of the hydrate.
 Source: chegg.com
Source: chegg.com
Find the formula and name of the hydrate. Find the formula of the hydrate. How can i determine the formula of a hydrate? This gives it a strong attraction toward ions. What is the formula of the hydrate?
 Source: odinity.com
Source: odinity.com
1 answer ramith hettiarachchi jun 18, 2014 this 10 minute video will help you ! When you react 3.9267 grams of na2co3 · nh2o with excess hcl(aq. A hydrate is an ionic compound that has a definite amount of water molecules attached to its crystalline structure. The formula of the hydrate shows the ratio of the moles of anhydrous salt to the moles of water. Find the formula of the hydrate.
 Source: youtube.com
Source: youtube.com
An example of a hydrate is cobalt(ii) nitrate hexahydrate, co(no3)2∙6h2o. From the calculation, you can clearly see that the units of g/mol in the numerator and denominator cancel out. Determining the formula of a hydrate. An example of a hydrate is cobalt(ii) nitrate hexahydrate, co(no3)2∙6h2o. A hydrate is an ionic compound that has a definite amount of water molecules attached to its crystalline structure.
 Source: slideserve.com
Source: slideserve.com
Determine the formula of the hydrate and then write out the name of the hydrate. Calculating amount of water in hydrate. To name a hydrate, we name the ionic compound followed by a greek prefix to indicate the number of water molecules ending in the word “hydrate.” (these are. The substance consisted of dull, compacted crystals. The steps to determining the.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title determining the formula of a hydrate by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.