Topic 3 the atomic structure
Topic 3 The Atomic Structure. Show how to configure the electronic configuration of atom; Atomic structure questions with answers ~ chemistry form two 2. Parts of the atom (subatomic particles) & reference table o T2.1_u2 negatively charged electrons occupy the space outside the nucleus.
 Topic 2 Atomic Structure Chemistry Punter From chemistrypunter.weebly.com
Topic 2 Atomic Structure Chemistry Punter From chemistrypunter.weebly.com
19 calculating particles in an atom. T2.1_u3 the mass spectrometer is used to determine the relative atomic mass of an element from its isotopic composition. T2.1_u1 atoms contain a positively charged dense nucleus composed of protons and neutrons (nucleons). Isotopes are atoms with the same atomic number, different mass numbers or the same number of protons, but different. Atomic structure flashcards from benjamin warwick�s wallace high school lisburn class online, or in brainscape�s iphone or android. Learn faster with spaced repetition.
Show how to configure the electronic configuration of atom;
Topic 3 atomic structure learning outcomes by the end of this topic, you should be able to: Learn vocabulary, terms, and more with flashcards, games, and other study tools. A subatomic particle that has a negative charge. Iodine atomic mass is 126.9044 so its mass number is 127. From its excited state to ground state of a lower energy level. Learn faster with spaced repetition.
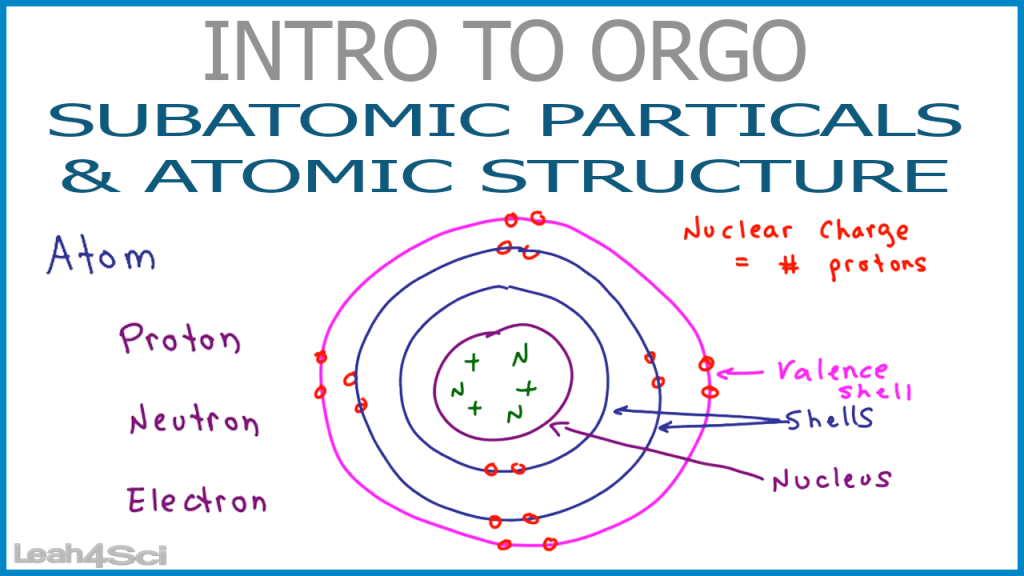 Source: leah4sci.com
Source: leah4sci.com
Learn vocabulary, terms, and more with flashcards, games, and other study tools. Differentiate between atomic number, nucleon number and mass number; Electron releases energy (emission) → the electron falls. Learn vocabulary, terms, and more with flashcards, games, and other study tools. Summarise the concepts of isotopes;
 Source: chemistrypunter.weebly.com
Source: chemistrypunter.weebly.com
Atoms of different elements have different numbers of protons and electrons. Iodine atomic mass is 126.9044 so its mass number is 127. Electron absorb energy (absorption) → the electron is. Mass number (a) = number of (protons + neutrons). Atomic structure flashcards from benjamin warwick�s wallace high school lisburn class online, or in brainscape�s iphone or android.
Source: dailymotion.com
T2.1_u2 negatively charged electrons occupy the space outside the nucleus. T2.1_u1 atoms contain a positively charged dense nucleus composed of protons and neutrons (nucleons). It is the fundamental characteristic of an element. Differentiate between atomic number, nucleon number and mass number; T2.1_u2 negatively charged electrons occupy the space outside the nucleus.
 Source: youtube.com
Source: youtube.com
Electron releases energy (emission) → the electron falls. F electron nearest to nucleus has the lowest energy and most. A subatomic particle that has a negative charge. Atomic number = 3 atomic mass = 6.941 7 = mass # # of protons = 3 # of electrons = 3 # of neutrons = 4 3 =4. Summarise the concepts of isotopes;
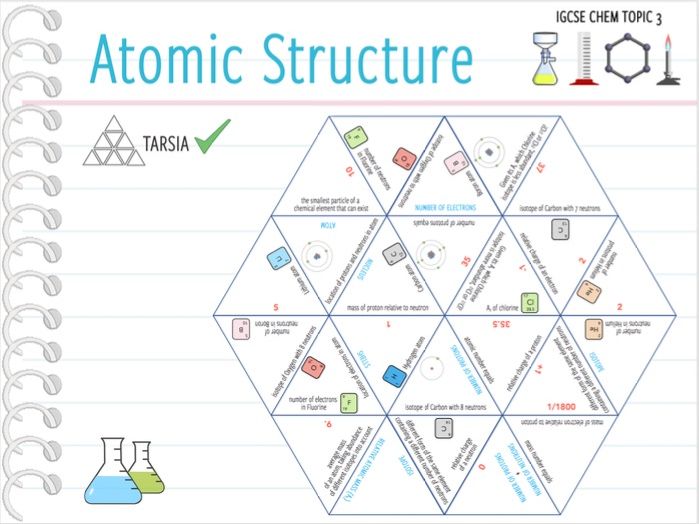 Source: tes.com
Source: tes.com
What is the atomic structure of a phosphorus atom? Topic #2 atomic structure study guide. Learn vocabulary, terms, and more with flashcards, games, and other study tools. Atomic structure learning outcomes by the end of this topic, you should be able to: A subatomic particle that has a positive charge and that is found in the nucleus of an atom.
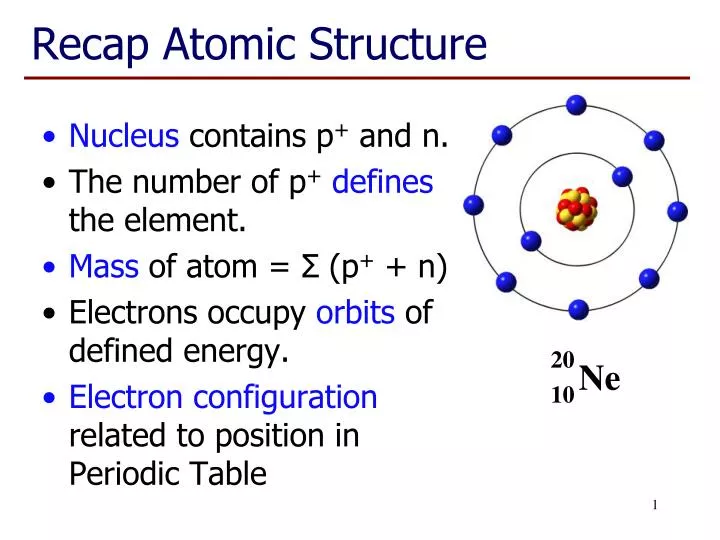 Source: slideserve.com
Source: slideserve.com
Learn vocabulary, terms, and more with flashcards, games, and other study tools. Explain the history of atomic model; Atoms of an element have identical numbers of protons and electrons, but can have different numbers of neutrons. T2.1_u1 atoms contain a positively charged dense nucleus composed of protons and neutrons (nucleons). Learn vocabulary, terms, and more with flashcards, games, and other study tools.
 Source: youtube.com
Source: youtube.com
Parts of the atom (subatomic particles) & reference table o Show how to configure the electronic configuration of atom; Atomic structure questions with answers ~ chemistry form two 2. Atomic number = 3 atomic mass = 6.941 7 = mass # # of protons = 3 # of electrons = 3 # of neutrons = 4 3 =4. Topic #2 atomic structure study guide.
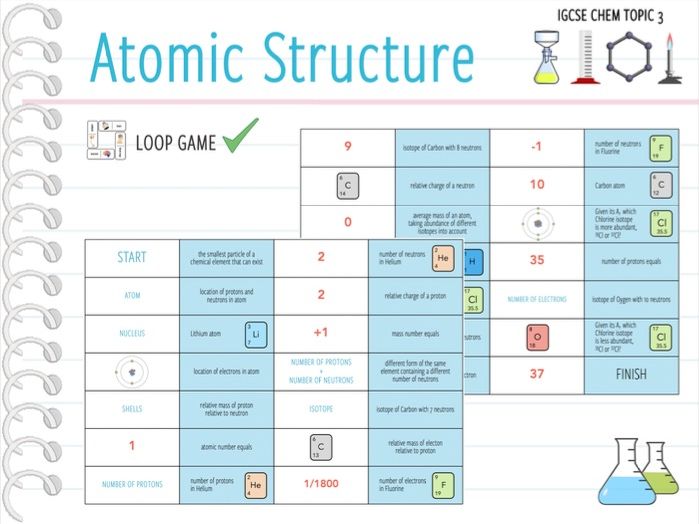 Source: tes.com
Source: tes.com
Isotopes are atoms with the same atomic number, different mass numbers or the same number of protons, but different. Iodine mass # 127 iodine atomic # 53 = 74 neutrons. Parts of the atom (subatomic particles) & reference table o Atomic structure atomic number (z) = number of protons. Isotopes are atoms with the same atomic number, different mass numbers or the same number of protons, but different.
 Source: youtube.com
Source: youtube.com
Mass # atomic # charge (if any) element. Parts of the atom (subatomic particles) & reference table o Stable state = ground state. Learn vocabulary, terms, and more with flashcards, games, and other study tools. Topic questions home / igcse / chemistry / cie / topic questions / 3.
 Source: youtube.com
Source: youtube.com
Atoms of an element have identical numbers of protons and electrons, but can have different numbers of neutrons. Electron absorb energy (absorption) → the electron is. History of atomic structure and the electrical nature of the atom and its particles involve key scientists including dalton, thomson, rutherford, bohr and others. Learn vocabulary, terms, and more with flashcards, games, and other study tools. A subatomic particle that has a positive charge and that is found in the nucleus of an atom.
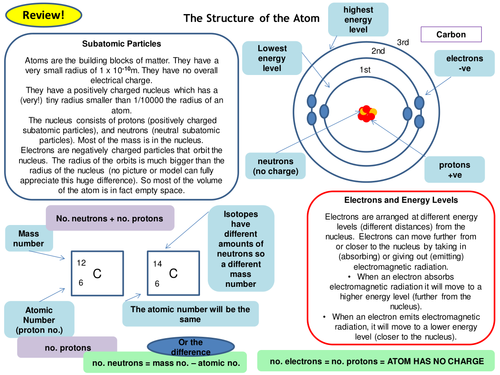
Mass number can be used to calculate neutrons in an atom. Summarise the concepts of isotopes; Topic #2 atomic structure study guide. Mass # atomic # charge (if any) element. Explain the history of atomic model;
 Source: leverageedu.com
Source: leverageedu.com
Elevated to a higher energy level (excitation) → excited state. From its excited state to ground state of a lower energy level. Isotopes are atoms with the same atomic number, different mass numbers or the same number of protons, but different. Electron absorb energy (absorption) → the electron is. F electron nearest to nucleus has the lowest energy and most.

Topic questions home / igcse / chemistry / cie / topic questions / 3. Atomic structure questions with answers ~ chemistry form two 2. Differentiate between atomic number, nucleon number and mass number; T2.1_u2 negatively charged electrons occupy the space outside the nucleus. Iodine atomic mass is 126.9044 so its mass number is 127.
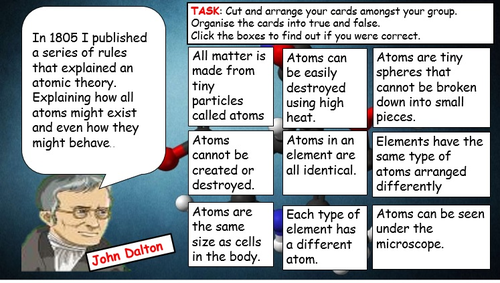
Parts of the atom (subatomic particles) & reference table o Parts of the atom (subatomic particles) & reference table o A subatomic particle that has a positive charge and that is found in the nucleus of an atom. Topic #2 atomic structure study guide. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
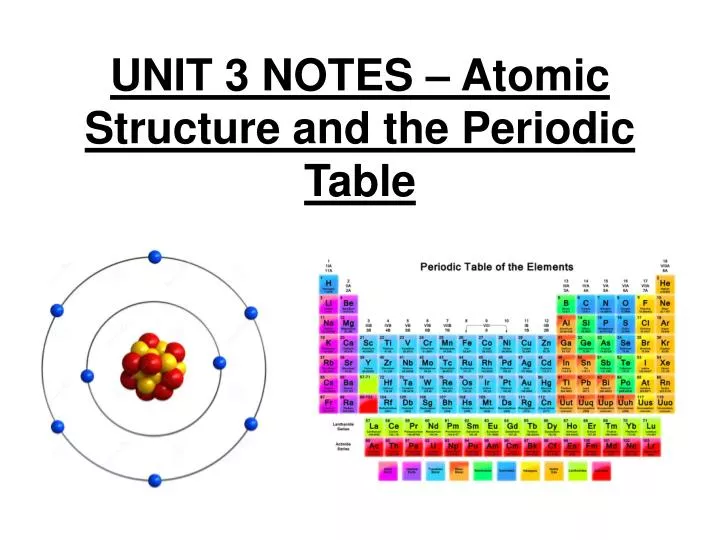 Source: brokeasshome.com
Source: brokeasshome.com
20 element # of protons # of. Learn vocabulary, terms, and more with flashcards, games, and other study tools. Mass # atomic # charge (if any) element. Differentiate between atomic number, nucleon number and mass number; Summarise the concepts of isotopes;
 Source: kikoro-designs.blogspot.com
Source: kikoro-designs.blogspot.com
One scientist’s discoveries leading the way for the next. 19 calculating particles in an atom. Mass number can be used to calculate neutrons in an atom. A subatomic particle that has a positive charge and that is found in the nucleus of an atom. Topic questions home / igcse / chemistry / cie / topic questions / 3.
 Source: tes.com
Source: tes.com
Topic #2 atomic structure study guide. Summarise the concepts of isotopes; Explain the history of atomic model; From its excited state to ground state of a lower energy level. Show how to configure the electronic configuration of atom;
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title topic 3 the atomic structure by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.




