What is the charge of an alpha particle
What Is The Charge Of An Alpha Particle. An alpha particle is made up of two protons and two neutrons bound together. It has a charge of +2. The production of alpha particles is termed alpha decay. They are used in smoke detectors, which is used as a fire indicator.
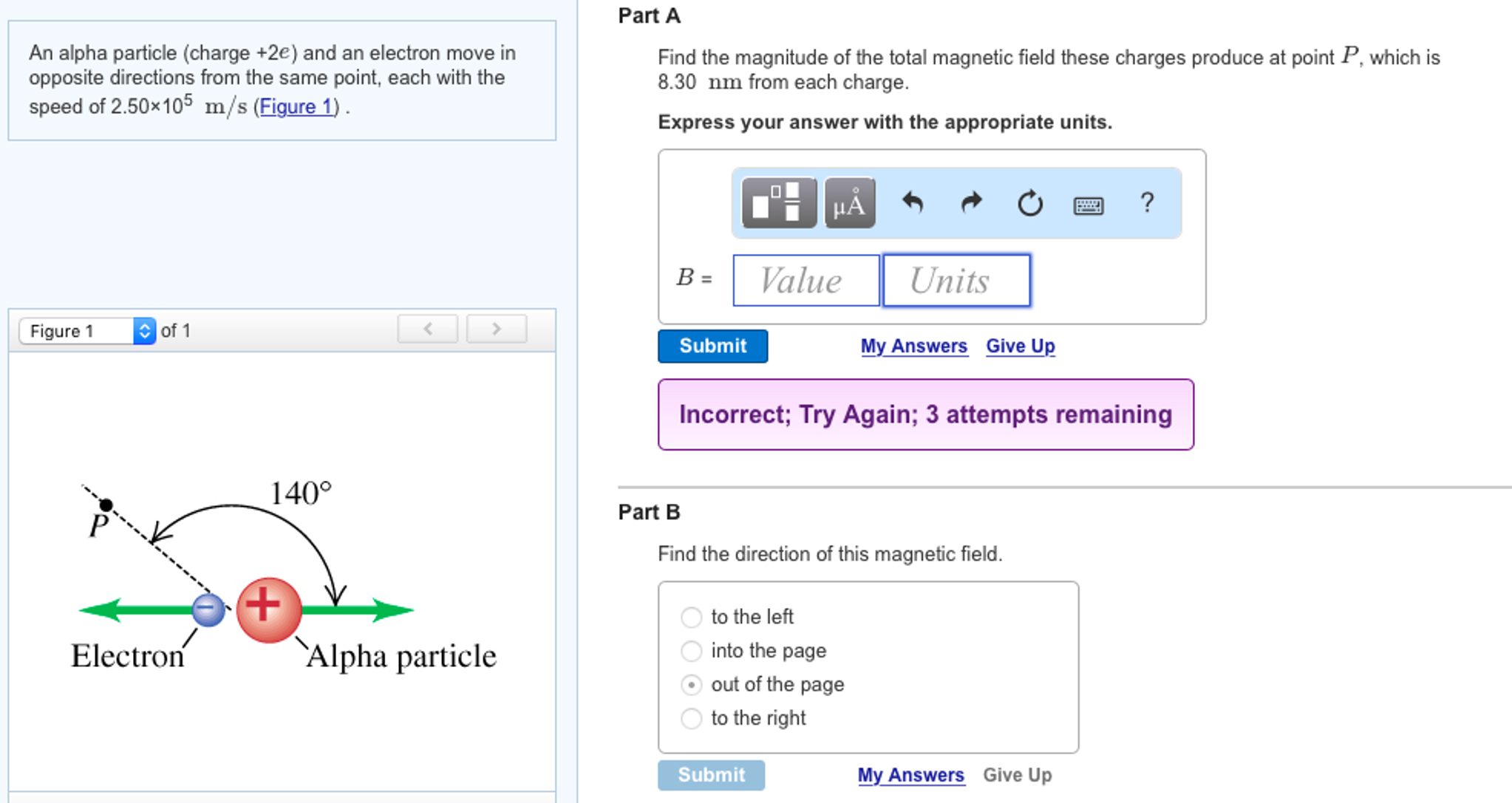 Solved An Alpha Particle (charge +2e) And An Electron Mov… From chegg.com
Solved An Alpha Particle (charge +2e) And An Electron Mov… From chegg.com
Alpha particles are relatively large and carry a double positive charge. An alpha particle consists of two protons and two neutrons. Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus. Hence, the charge on an alpha particle is equal to 3.2 × 10 − 19 c. Represented by greek alphabet α. What is the charge of an alpha particle?
So the charge on an alpha particle is given by.
(the above two properties prove that an alpha particle is equal to the helium atom which has lost its two orbital. Gamma rays are waves of electromagnetic energy, or photons. They are not very penetrating and a piece of paper. Beta particles are high energy electrons. They are used in smoke detectors, which is used as a fire indicator. One kind of radiation is a particle of matter, called the alpha particle.
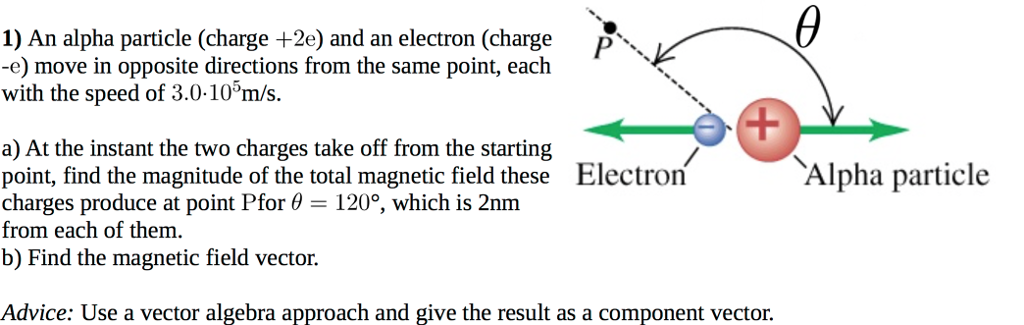 Source: chegg.com
Source: chegg.com
Represented by greek alphabet α. Alpha particles have a charge of +2,hence, charge on an alpha particle is twice the electronic charge =2×1.6×10 −19cq=3.2×10 −19c. It has a charge of +2. Alpha particles carry double the positive charge of the proton, i.e., equal to the charge on the helium nucleus. What are the properties of alpha beta and gamma particles?
 Source: numerade.com
Source: numerade.com
Alpha particles carry a positive charge, beta particles carry a negative charge, and gamma rays are neutral. Alpha particles carry double the positive charge of the proton, i.e., equal to the charge on the helium nucleus. Alpha particles (α) are positively charged and made up of two protons and two neutrons from the atom’s nucleus. Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus. What is the charge of an alpha particle?
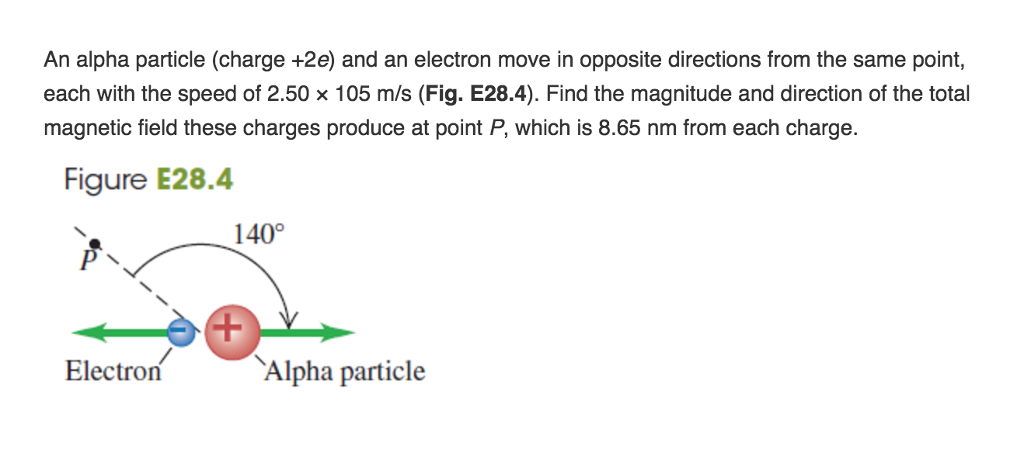 Source: chegg.com
Source: chegg.com
Alpha particles are relatively large and carry a double positive charge. Hence, the charge on an alpha particle is equal to 3.2 × 10 − 19 c. They are used in smoke detectors, which is used as a fire indicator. The alpha particles discussed in this question are used in many day to day applications. Alpha particles are energetic nuclei of helium.
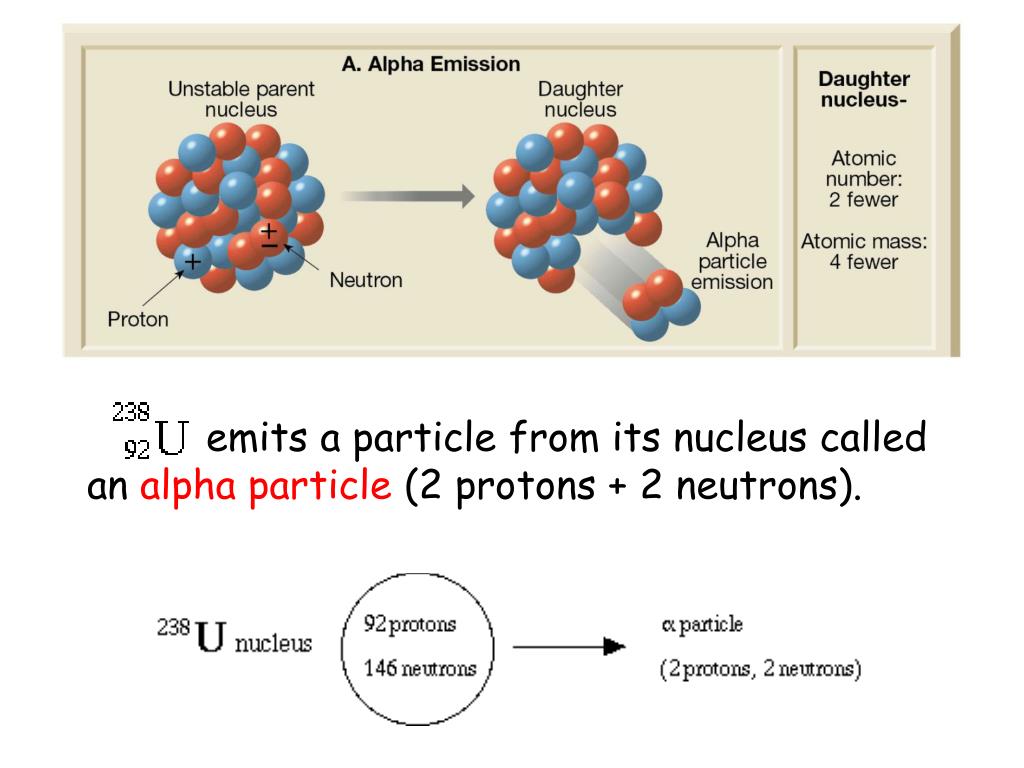 Source: slideserve.com
Source: slideserve.com
So the charge on an alpha particle is given by. It is a doubly charged helium nucleus. The charge of an alpha particle is 2 positive. Beta particles are emitted from nuclei undergoing radioactive decay, and are either electrons with a. It is a positively charged particle emitted from the decay of various radioactive materials.
 Source: chegg.com
Source: chegg.com
Alpha particles come from the decay of the heaviest radioactive elements, such as uranium, radium and polonium. Alpha particles carry double the positive charge of the proton, i.e., equal to the charge on the helium nucleus. Alpha particles come from the decay of the heaviest radioactive elements, such as uranium, radium and polonium. An alpha particle consists of two protons and two neutrons. Alpha particles have a charge of +2,hence, charge on an alpha particle is twice the electronic charge =2×1.6×10 −19cq=3.2×10 −19c.
 Source: doubtnut.com
Source: doubtnut.com
(the above two properties prove that an alpha particle is equal to the helium atom which has lost its two orbital. Hence option c is the answer. What are the properties of alpha beta and gamma particles? Thus, an alpha particle contains two protons and two neutrons. They are not very penetrating and a piece of paper.
 Source: doubtnut.com
Source: doubtnut.com
Alpha particles (α) are positively charged and made up of two protons and two neutrons from the atom’s nucleus. Gamma rays are waves of electromagnetic energy, or photons. Beta particles are high energy electrons. Hence, the charge on an alpha particle is equal to 3.2 × 10 − 19 c. An alpha particle is made up of two protons and two neutrons bound together.
 Source: cronodon.com
Source: cronodon.com
The most energetic alpha particle will generally fail to. A single particle�s mass is 4 amu (6.642×10−4 g),. Beta particles are emitted from nuclei undergoing radioactive decay, and are either electrons with a. Which particle is lighter than others? An alpha particle is made up of two protons and two neutrons bound together.
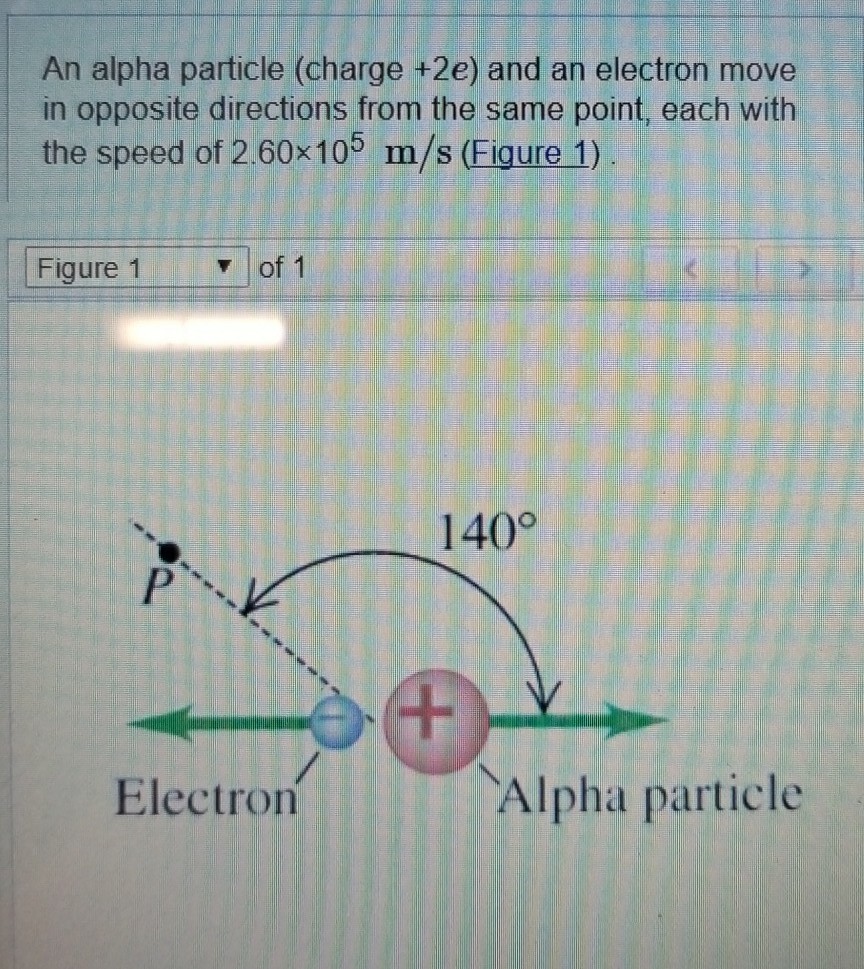
One kind of radiation is a particle of matter, called the alpha particle. 0 (0) (1) (0) choose an option that best describes your problem. A single particle�s mass is 4 amu (6.642×10−4 g),. An alpha particle is made up of two protons and two neutrons bound together. A positively charged particle ejected spontaneously from the nuclei of some radioactive elements.
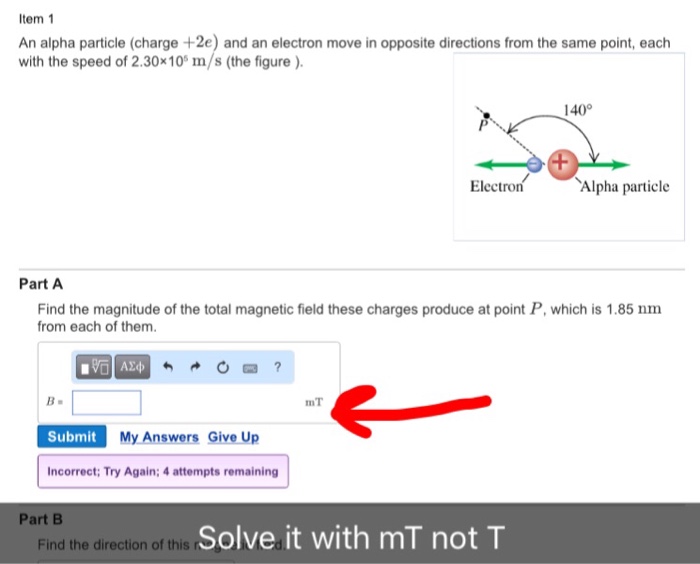
So the charge on an alpha particle is given by. Gamma rays are waves of electromagnetic energy, or photons. It is a doubly charged helium nucleus. The production of alpha particles is termed alpha decay. Hence option c is the answer.
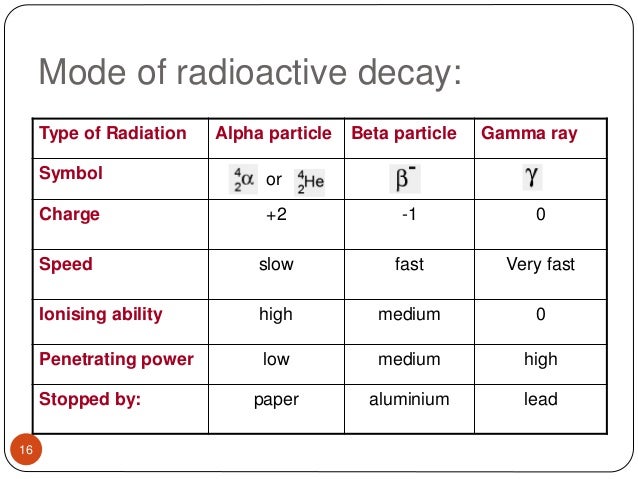 Source: slideshare.net
Source: slideshare.net
What is the charge of an alpha particle? The charge of an alpha particle is 2 positive. The production of alpha particles is termed alpha decay. What kind of electric charge did the alpha particles have? Charge on an alpha particle is twice the charge of an electron.
 Source: chernobylguide.com
Source: chernobylguide.com
A single particle�s mass is 4 amu (6.642×10−4 g),. An alpha particle is made up of two protons and two neutrons bound together. An alpha particle is identical to a helium atom that has been stripped of its two electrons; Specific charge of an alpha particle is the ratio of charge on alpha particle to the mass of it. 0 (0) (1) (0) choose an option that best describes your problem.
 Source: amp.doubtnut.com
Source: amp.doubtnut.com
It has a charge of +2. Hence option c is the answer. An alpha particle consists of two protons and two neutrons. 0 (0) (1) (0) choose an option that best describes your problem. What is the charge of an alpha particle?
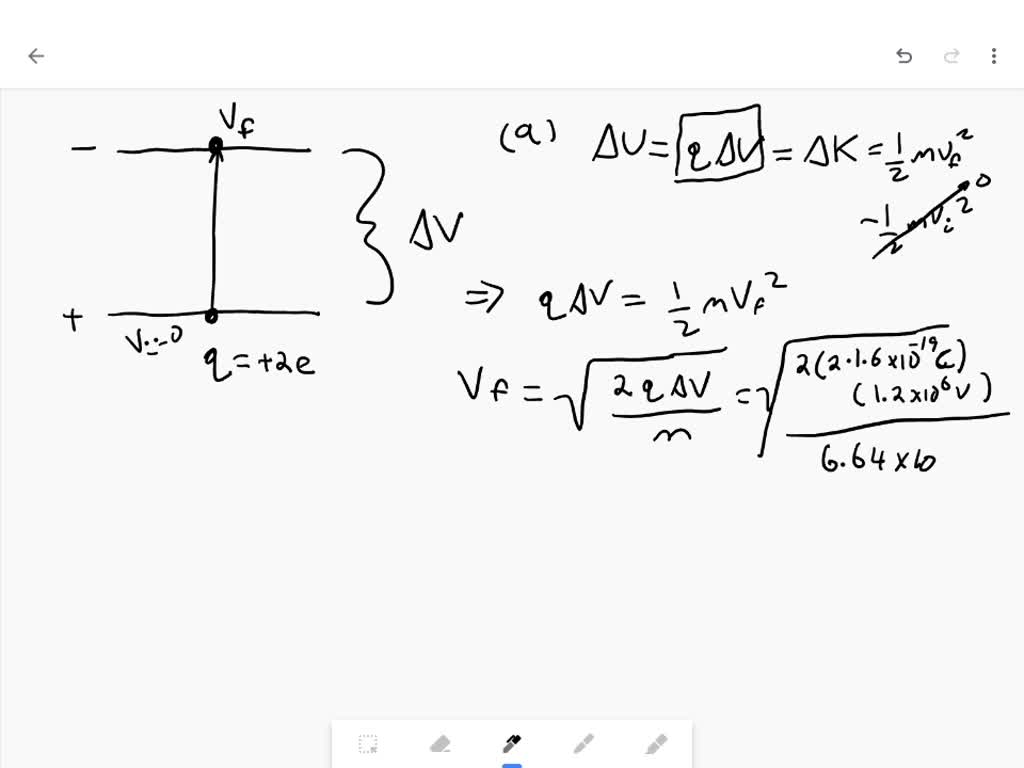 Source: numerade.com
Source: numerade.com
Alpha particles are energetic nuclei of helium. Beta particles are high energy electrons. Alpha particles carry a positive charge, beta particles carry a negative charge, and gamma rays are neutral. It is a positively charged particle emitted from the decay of various radioactive materials. Alpha particle is alternatively known as alpha radiation or alpha ray.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
An alpha particle is identical to a helium atom that has been stripped of its two electrons; It is a doubly charged helium nucleus. Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus. They are not very penetrating and a piece of paper. They are used in smoke detectors, which is used as a fire indicator.
 Source: youtube.com
Source: youtube.com
Alpha particle is alternatively known as alpha radiation or alpha ray. Alpha particles are relatively large and carry a double positive charge. One kind of radiation is a particle of matter, called the alpha particle. Specific charge of an alpha particle is the ratio of charge on alpha particle to the mass of it. Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus.
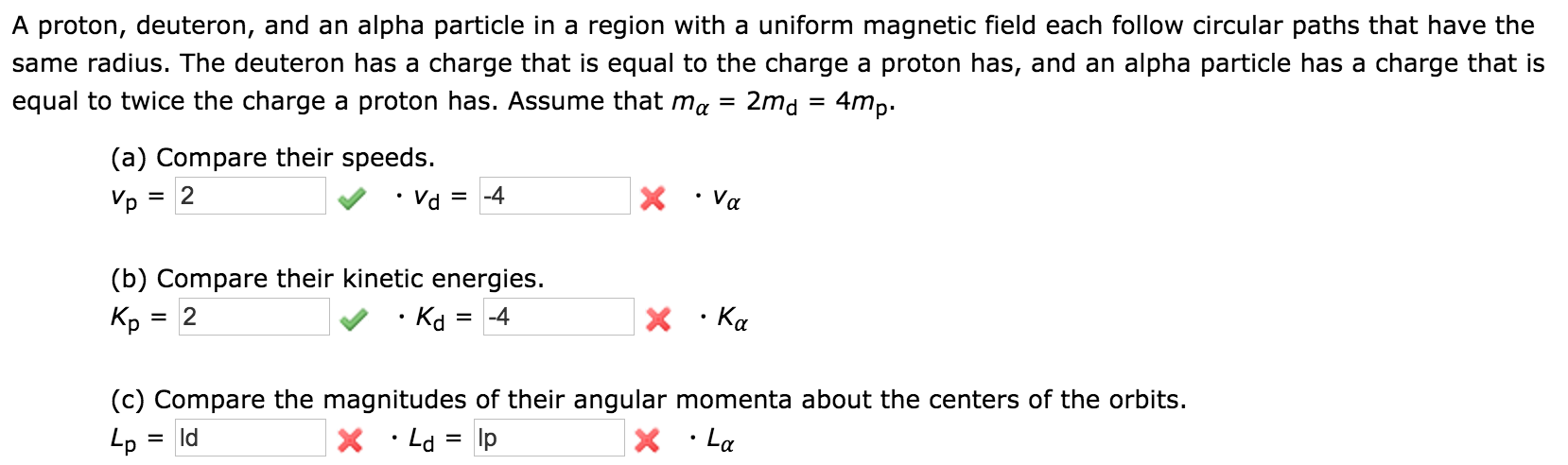 Source: chegg.com
Source: chegg.com
It has a positive electric charge and about four times the mass of a hydrogen atom. Alpha particles come from the decay of the heaviest radioactive elements, such as uranium, radium and polonium. Alpha particle mass is due to the two protons and two neutrons bonding. The production of alpha particles is termed alpha decay. An alpha particle is made up of two protons and two neutrons bound together.
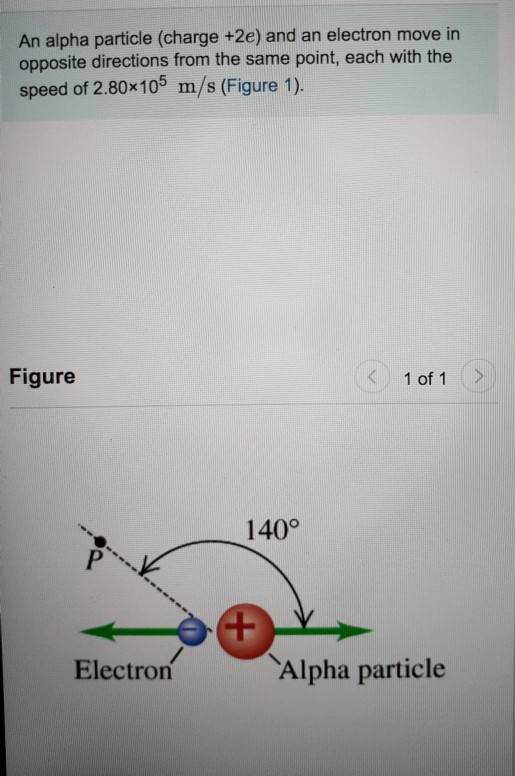
Alpha particles are positively charged particles that comprise two protons, two neutrons, and zero electrons. They are not very penetrating and a piece of paper. What are the properties of alpha beta and gamma particles? Alpha particles are energetic nuclei of helium. It has a charge of +2.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title what is the charge of an alpha particle by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





